Introduction
Materials and Methods
Chemicals
Plant material
Standard Curve Preparation
Precision, Accuracy and Library Match test
HPLC Analysis
LC-MS analysis
Results and Discussion
Introduction
Toxicity assessment, standardization of marker substances, preclinical evaluation, and clinical trials are essential for industrialization with natural pharmaceuticals, functional foods, and functional cosmetics. The quality control on natural products is principal key for the industrialization of functional foods, cosmeceuticals and pharmaceuticals using the natural resources. Moreover, company and researchers handling natural substances have been required the strict QC method for the Good Manufacturing Practices (GMP) and Good Clinical Practices (GCP). Quality control of natural products is used as an indicator to guarantee the functionality of natural products. To set up analytical methods of natural products are difficult due to the complexity of the components, hence appropriate methods for quality control are required.
We have been conducting research to establish analytical methods of single plants and herbal preparations such as Coptis chinensis (Yu et al., 2017), Gami-Samhwang-San (Yu et al., 2005a) and Gami-Honghwa-Tang (Yu et al., 2005b; Yu et al., 2004) by HPLC. Furthermore, we reported the analysis method of Samul-tang (Yu et al., 2007a, b) and Sipjeondaebo-tang fomula (Shin et al., 2011) by HPLC-MS-MS. Scutellaria baicalensis GEORGI is a perennial growing to 0.3 m in the Labiatae family (Kim et al., 2014). This plant inhabits Shandong, Sìchuan in Chinese, and entire region in Korea and is in flower in from June to August, and the seeds ripen in September. (Korea National Arboretum, 2014). According to Korean Pharmacopoeia 9th edition, Scutellaria Root is cone-shaped, semitubular or flattened root, 5 ㎝ to 25 ㎝ in length, almost odorless, and it has slightly bitter taste.
Scutellariae Radix is characterized by bitterness and coldness and acts on the lung, stomach and intestine channels (Yang et al., 2013). In the traditional Korean medicine, bitterness dries dampness, and coldness clears heat (Bensky et al., 2004). Studies on the pharmacological activity of ginseng have been carried out in various clinical and preclinical fields. The biological activities of Scutellaria Radix have been reported on melanin inhibition (Kim et al., 2012), anti-proliferation (Chui et al., 2005), hepatoprotective effect (Jang et al., 2003), anti-oxidation and neuro-protective activity (Lee et al., 2014), anti-hypertensive effect (Song et al., 2013), anti-inflammation (Chi et al., 2001; Chi and Kim, 2005; Chi et al., 2003; Kim et al., 2009), anti-virus (Moghaddam et al., 2014), anti-allergy (Hsieh et al., 2007), and anti-bacterial effect (Yun et al., 2012).
In the physicochemical study of secondary metabolites, oroxylin-A (Ku et al., 2014; Li and Chen, 2005), baicalein (Horvath et al., 2005), chrysin (Tong et al., 2012), skullcapflavone (Jang et al., 2012), oroxylin-α-7-O-glucuronide, wogonin, wogonoside (Wu et al., 2005), and baicalin (Ohkoshi et al., 2009) were isolated from chromatographical method and elucidated by spectroscopic method.
HPLC, UPLC, HPLC-MS, and HPLC-MS-NMR methods have been reported for quantitative and qualitative analysis of Scutellaria radix (Bardakci et al., 2018; Liu et al., 2014; Yang et al., 2018; Zhang et al., 2018). In this study, we tried to find a more efficient and easy analytical method of marker substances of Scutellaria radix. For this purpose, we isolated and elucidated 4 major flavonoids such as baicalin, baicalein, wogonoside, and wogonin (Yu, 2014). This study presents quantitative analysis of baicalin using the high performance liquid chromatography-photodiode array detector (LC-DAD) and provides high performance liquid chromatography-electrospray ionization-mass spectrometer (LC-ESI/MS) methods to identify various flavonoids such as wogonoside, baicalin, and wogonin in Scutellariae Radix.
Materials and Methods
Chemicals
The baicalin, baicalein, wogonoside, and wogonin were directly isolated from the Scutellariae Radix. There chemical structures were elucidated by spectroscopic analysis (Yu, 2014). The HPLC grade solvent used for the mobile phase (Acetonitrile and methanol), and other chemicals were purchased from Sigma (St. louis, MO, USA).
Plant material
The roots of S. baicalensis (Scutellariae Radix) were collected from Yeosu in Jeollanamdo, South Korea. Scutellariae Radix was identified through its morphological structures and sensory evaluation such as appearance, taste, odor, and texture. A voucher specimen (NBU-HP-S-032) of the plants was deposited in the herbarium of the Nambu University, Gwangju, Korea.
Standard Curve Preparation
To prepare a test solution, the pulverized Scutellaria Root was accurately weighed 0.5 g, added 30 ㎖ of mobile phase (d-phosphoric acid:CH3CN, 18:7), heated under a reflux condenser in a water-bath for 30minute, and filtered. To the residue, added 30 ㎖ of mobile phase (d-phosphoric acid:CH3CN, 18:7) and proceeded in the same manner. Combined all the extracts, add 40 ㎖ of mobile phase (d-phosphoric acid:CH3CN, 18:7) to make exactly 100 ㎖ and use this solution as the test solution. Separately, weighed accurately each of about 10 mg of baicalin (previously dried in a desiccator in vacuum at a pressure not exceeding 0.67 kPa (silica gel) for not less than 24 hours) and dissolved in methanol to give various concentrations within the range 3.5-175 ㎍/㎖, respectively. The whole volume of standard solution mixture is 1 ㎖. 20 ㎕ aliquots of test solution and baicalin were injected for analysis.
Precision, Accuracy and Library Match test
To validate the precision (reproducibility), the test was repeated 3 times with 10 ㎕ of the standard solution under the HPLC operating conditions. To confirm the accuracy (recovery test), a proper amount of test solution was allocated in to five portions (one as a control group), each portion (except the control) was spiked with diverse concentrations of standard solution to add several concentrations of baicalin. All samples were injected for HPLC analysis to analyze the recovery. We conducted the library matching test to identify peaks by comparing spectra from unknown peaks to spectra from standards.
HPLC Analysis
The HPLC system consisted of a multi-solvent delivery pump (Waters 2690, USA), and a diode-array UV/Vis multi-wavelength detector (Waters 996, USA). The peak signals from the detector were collected and calculated with a computer equipped with a software Millennium 4.0. The separation was carried out on C18 reversed-phase column (particle size 5 ㎛, 4.6x250 ㎜ i.d., X-terra, Waters, USA). The mobile phase was composed of 0.68% phosphoric acid (62%) and acetonitrile (38%) with isocratic elution. The solvents were filtered through a 0.45 ㎜ Millipore filter and degassed prior to use. The flow rate was 1 ㎖/min with DAD scanning at 277 ㎚ (2D) and 200-550 ㎚ (3D). The operating temperature was maintained at 50℃.
LC-MS analysis
LC-ESI/MS was performed using a ZQ Mass spectrometer equipped with an atmospheric pressure interface electrospray chamber using interfaced to a Waters Alliance 2695 series liquid chromatograph. Waters MassLynx 4.0 was used for data collection and analysis. The separation of compounds was carried out on C18 reversed-phase column (Xterra Ms C18 column, Waters, MA, USA). The flow rate was 0.2 ㎖/min. Two solvents, solvent A (A=0.05% TFA in water) and solvent B (B=0.05% TFA in CH3CN) were used in this method. The linear gradient elution of the solvents was programmed as follows: 0-30min(10→30% B), 30-35min(30 → 30% B), 35-50min (30 → 90% B), 50-55 min (90 → 90% B), 55-60 min (90 → 10% B). The conditions for ESI-MS analysis in both positive and negative ion mode included a cone voltage of 20, 40 and 60 V, a capillary voltage of 3.2 kV, an extractor of 5.0 V, a RF lens of 0.5V, a desolvation gas flow of 250 L/hr, a cone gas flow of 50 L/hr., a desolvation temperature of 170℃ and a source temperature of 120℃.
Results and Discussion
The analysis of the interesting compounds in complex natural mixtures requires sophisticated hyphenated techniques, which should provide good selectivity and sensitivity. With the development of analytical instruments, we have been able to acquire information on complex and trace amounts of components more easily. The combination of HPLC and Photodiode Array Detection (LC-DAD) allows us to obtain structural information for complex mixtures. The HPLC-mass spectrometry (Mathon et al., 2014) and nuclear magnetic resonance (LC-NMR) (Brkljaca and Urban, 2014, 2015; Kasote et al., 2017) have made possible the acquisition of on-line complementary spectroscopic data on interesting peak in the natural products. These new-hyphenated techniques have been rapidly integrated for the study of crude plant extracts (Salman et al., 2016).
In this study, we employed a method, which can effectively determine the baicalin of Scutellariae Radix by HPLC. And we presented identifying method of various flavonoids such as wogonoside, baicalin, and wogonin in Scutellariae Radix by high performance liquid chromatography - electrospray ionization-mass spectrometer (LC-ESI/MS).
The HPLC method validation means evaluating the performance parameters of the method including the accuracy, precision, system suitability, specificity or selectivity, linearity, limit of detection and limit of quantification. The linearity test give assurance that this method is valid for their intended use throughout the specified range. The standard mixtures were injected in triplicate and the response factors were calculated for the validation of accuracy, precision, and linearity. The results were calculated relative standard deviation (R.S.D) of precision, accuracy, and linearity by Microsoft Excel 2016 program.
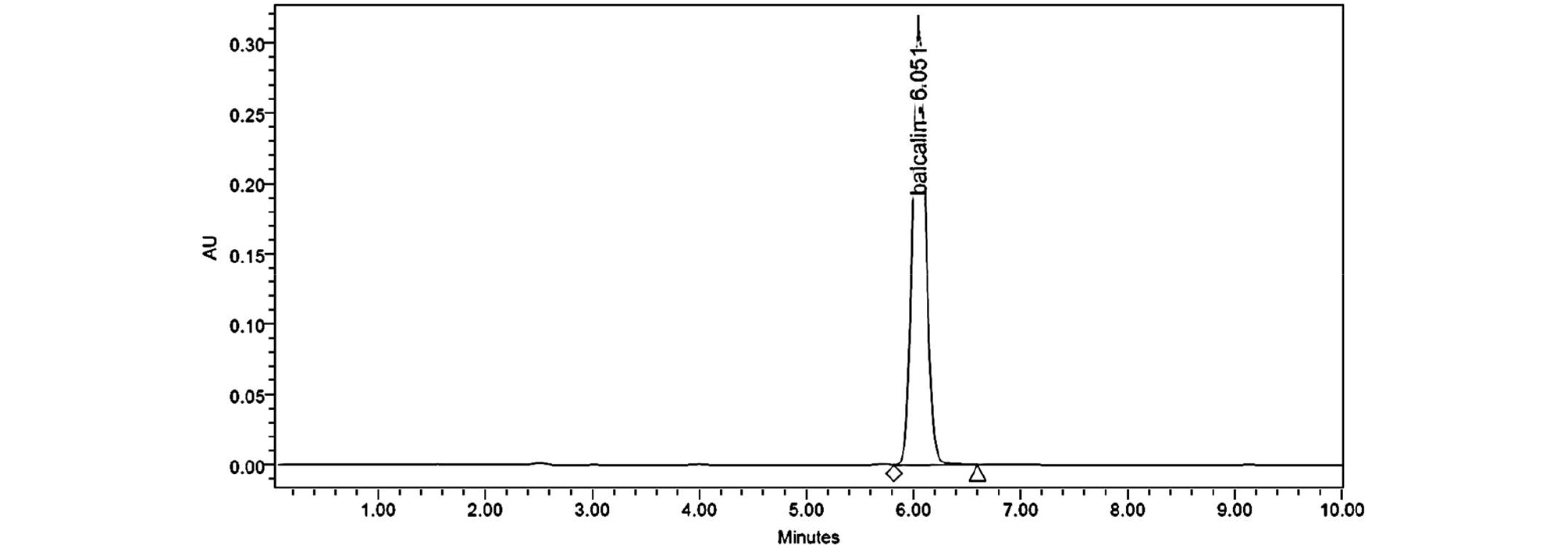
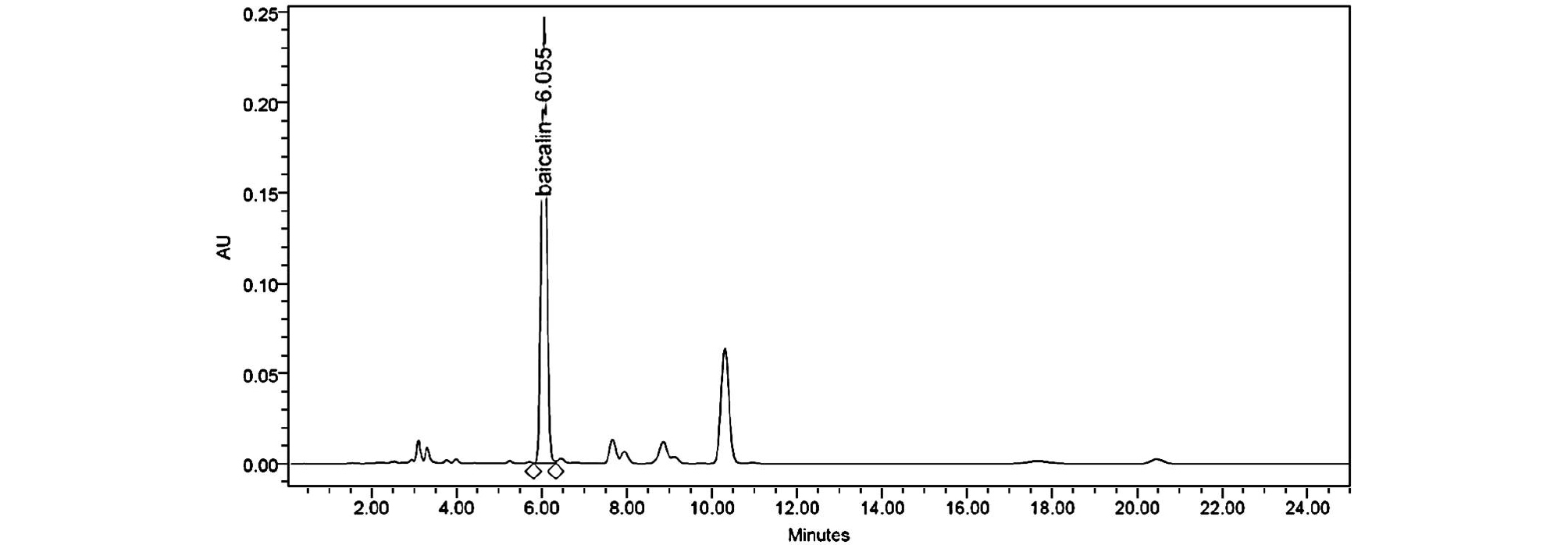
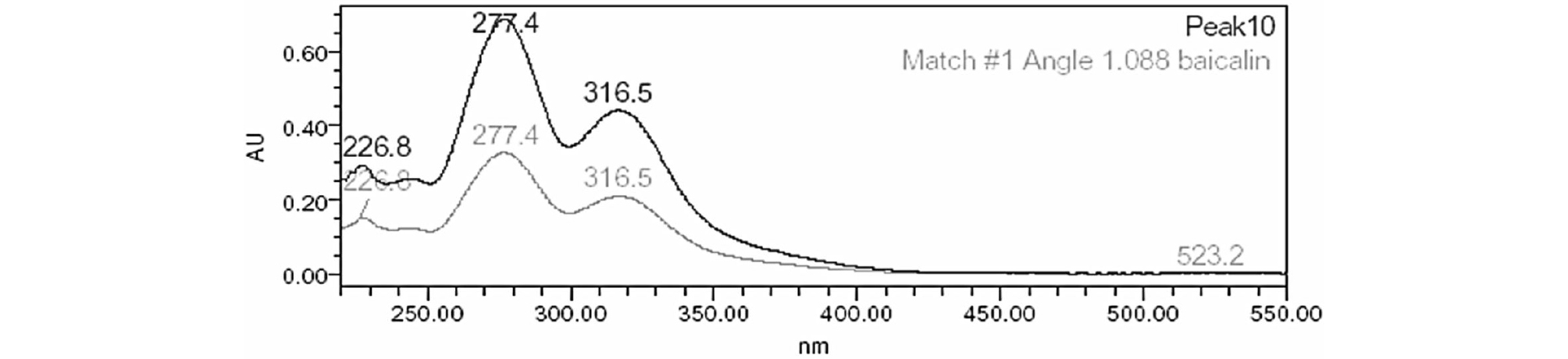
The regression equations of the graphs for baicalin was given y = 44150.05x -29123.1. Calibration graphs for baicalin were obtained over the ranges 3.5-175 ㎍/㎖. The correlation coefficients (R2) value is greater than 0.9999, indicating their acceptability. We injected standard solutions of baicalin at the concentrations of 3.50 ㎍/㎖, three times on the same day to check the precision of this method. Based on peak-area ratios for three replicate injections, the Inter-day relative standard deviations (R.S.D.s) were 0.92%. The inter day R.S.D.s obtained for a 5-day period were 1.23%, respectively. In all instances the accepted criteria of % RSD of less than 1.5% was met (Table 1). Accuracy of the method was conducted by recovery investigation. The recovery values were found to meet the acceptance criteria of 83-85% at different concentration levels. Table 2 indicated acceptable precision and accuracy for appropriate analysis method. Fig. 1 showed the chromatogram (277 ㎚) of baicalin and Fig. 2 showed the chromatogram of Scutellariae Radix extracts acquired the HPLC-DAD system. The amount of baicalin in Scutellariae Radix extracts was calculated as 10.46% through the HPLC quantitative analysis. We established a library of baicalin spectra (200~550 ㎚) obtained from HPLC-DAD. The baicalin spectrum in the Scutellariae Radix extracts was coincided by comparing from the HPLC retention time and UV-visible spectra of standards in the library (Fig. 3). The baicalin spectrum in the Scutellariae Radix extracts showed 1.088 of Purity Angle and 2.055 of Purity Threshold. Purity Angle is a new technology, which can test the purity of every peak of a compound and library match. If the Purity Angle is less than the Purity Threshold, the peak is spectrally homogeneous. Baicalin was quantitatively analyzed by HPLC-DAD. However, there are many other ingredients that need to be confirmed in Scutellariae Radix. We need more certain the chemical information of various compounds in Scutellariae Radix.
Table 1. Inter-day and intra-day relative standard deviations of baicalin (reproducibility) (n=3)
| Marker substance | Concentration (㎍/㎖) | Peak area of marker substance | R.S.D (%) | ||
| Inter-day | Intra-day | Inter-day | Intra-day | ||
| Baicalin | 3.50 | 137977.87 | 137936.25 | 0.92 | 1.23 |
Table 2. Relative recovery test of baicalin (n=3)
| Marker substance | Added (㎍/㎖) | Found (㎎) | Relative Recovery (%) | Mean±S.D. (%) | R.S.D (%) |
| Baicalin | 17.5 | 14.52 | 82.97 | ||
| 24.5 | 20.65 | 84.29 | |||
| 31.5 | 25.98 | 82.49 | |||
| 38.5 | 32.62 | 84.74 | 83.62±1.06 | 1.27 |
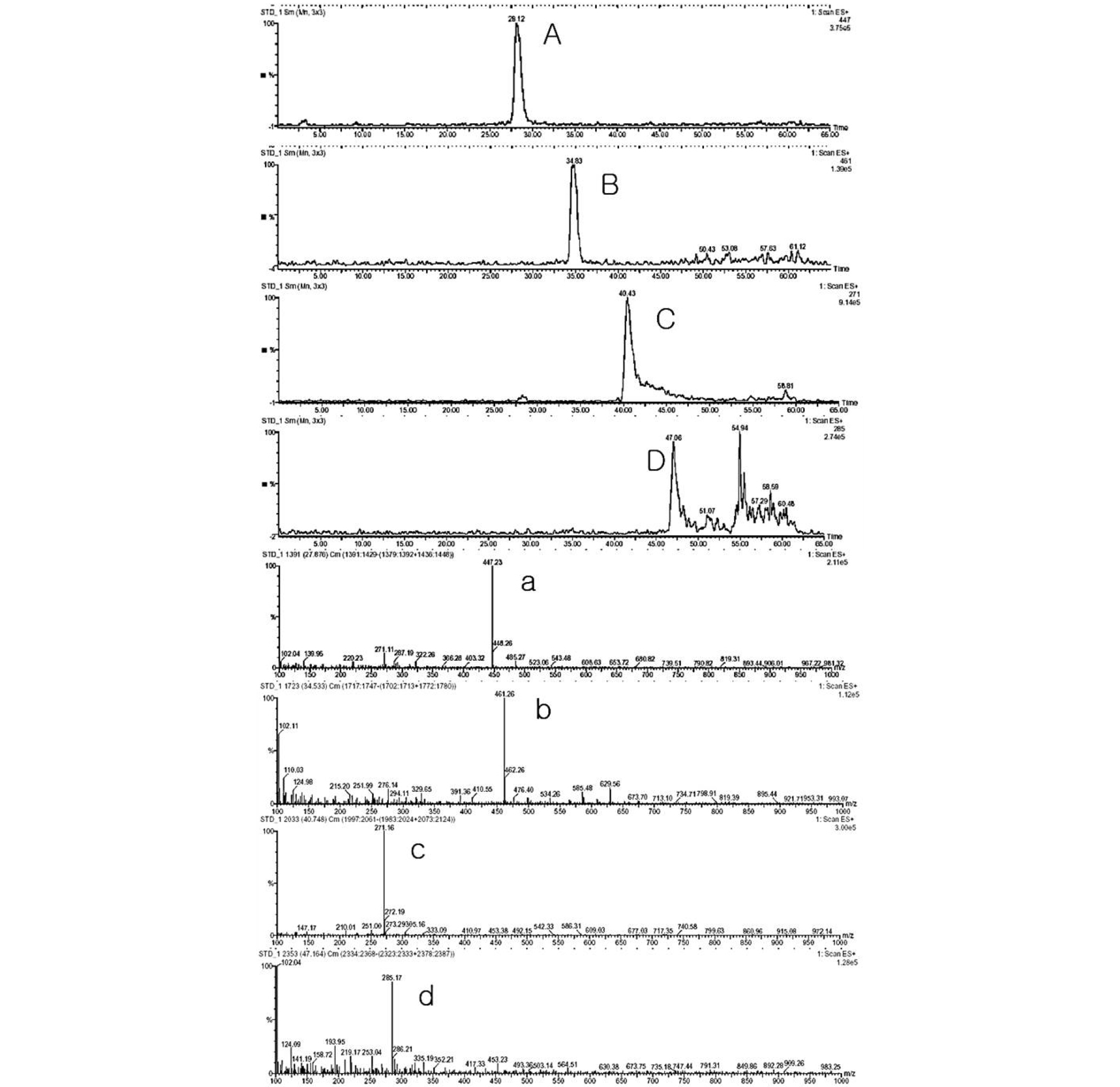
Therefore, we conducted the qualitative analysis the Scutellariae Radix by LC-ESI-MS. The results showed that baicalin, wogonoside, baicalein, and wogonin were always detected as the base peaks in the positive ion mode. ESI/MS spectra of compounds are presented in Fig. 4. The flavonoids of Scutellariae Radix showed a strong base peak [M]+ in the positive detection mode following as; baicalin (m/z; 271 [MH+-sugar]+, 447 [M+H]+), wogonoside (m/z; 285 [MH+-sugar]+, 461 [M+H]+), baicalein (m/z; 271 [M+H]+), wogonin (m/z; 285 [M+H]+). These results were coincided with fragment pattern and molecular weight of standard components and literature (He et al., 2016; Shi et al., 2011; Yang et al., 2018).
As a result, we identified marker substance, baicalin in Scutellariae Radix through quantitative and qualitative analytical method by HPLC-DAD. In addition, we can have assigned the tentative structures such as baicalin, wogonoside, baicalein, and wogonin in Scutellariae Radix by HPLC-ESI-MS method. We have been studying the standardization and identification of bioactive components from the traditional Korean medicine. This study on standardization of Scutellariae Radix would be provide the partial data of the scientific evidence of natural products.