Introduction
Materials and Methods
Cell culture
Red Charm Extracts
Reagents
DPPH radical scavenging assay
Nitric Oxide Assay
MAP Kinase (ERK) phosphorylation assay
MTT cell proliferation assay
Statistics
Results and Discussion
Effects of paeoniflorin and oxypaeoniflorin on cell viability of RAW 264.7 macrophages
Effects of oxypaeoniflorin and paeoniflorin on Radical Scavenging Activity in RAW264.7 macrophage cells
Effect of paeoniflorin and oxypaeoniflorin on Nitric Oxide production
Effect of oxypaeoniflorin and paeoniflorin on MAP Kinase phosphorylation
Introduction
Paeonia lactiflora has been one of the traditional medicinal herbs widely used in Asia for many years (Ahn et al., 2018; Bang et al., 1999; He and Dai, 2011; Mao et al., 2008; Park et al., 2009; Soka, 1985; Zheng et al., 2019; Zhu et al., 2018). Paeonia ‘Red Charm’ is soft-stemmed peony hybrid of P. lactiflora and P. officinalis. It is a shrubby perennial that will display attractive foliage in mid-spring throughout the summer and autumn and gives red double blooms. The root extracts of Paeonia lactiflora (P. lactiflora) cv. ‘Red Charm’ has been studied by many research groups, however little attention has been paid to its flower petal. Compared to functional aspects of the roots of P. lactiflora, very little attention has been given to those of its flower petals. To determine the detailed components of flower petals of P. lactiflora, we conducted the Fourier transform ion cyclotron resonance (FT-ICR) MASS spectrophotometric analysis. This analysis identified the twenty-four different types of components from the ethanol extracts of the flower petals of P. lactiflora cv. ‘Red Charm’. The main compounds were kaemperol glucopyranosides, quercetin glucopyranosides, paonioflolol and methyl gallate (Kim et al., 2016). We therefore further examined its functional activity of extracts. First, we examined anti-oxidant activity and the radical scavenging activity with 2,2-diphenyl-1-picrylhydrazyl (DPPH) of the P. lactiflora extracts was 87.9-90.4% at 0.1 mg/mL. This result showed that these flower petal extracts have approximately 5-fold stronger anti-oxidant activity than a previous report with those of root (Bang et al., 1999). The result of tyrosinase inhibition assay of P. lactflora petal extract was similar to those of arbutin, a well-known standard except significantly higher effect in the coral sunset extract at 0.1% concentration. Hyaluronidase inhibition assay also showed 76.5% inhibition at 5% concentration of this flower extract, indicating that P. lactiflora flower extracts have the major anti-oxidant, anti- inflammatory and brightening effects (Kim et al., 2016). Several studies have reported that the ethanol extract of the root parts commonly called peonies exhibit anti-allergic and painkilling effects (Bang et al., 1999; He and Dai, 2011; Lee et al., 2014; Mao et al., 2008; Park and Chun, 2015). This root has been recognized important for its use as a medicinal herb, but it is not the only part of the flower that has proven to be helpful. Polyphenol has been known to play an important role as an anti-inflammatory and antioxidant active ingredient from peony flower petal extracts (Jung et al., 2010). It is highly likely that the peony flower petal extracts contain a large amount of alkaloids kemperol glycosides and quercetin glycosides, which are presumed to have anti-oxidant and anti-inflammatory properties (Kim et al., 2016). In particular, quercetin has recently been reported to have diverse functional properties such as anti-inflammatory, antioxidant and anti- allergic properties (Mlcek et al., 2016).
Paeoniflorin, one of the main bioactive components of peony, has been shown to facilitate vasodilation and blood circulation, to strengthen the elastic cell binding force of smooth muscle cells in skeletal muscles, to relax muscle spasms, to enhance gastrointestinal peristalsis, and to improve digestion and absorption (Cheng et al., 1999). We previously showed that in addition to root part, the petal of P. lactiflora also has anti-oxidant and anti-inflammatory activity (Kim et al., 2016). Previous studies showed that paeoniflorin and oxypaeoniflorin are two of the main monoterpene glycosides of peony and can be extracted from the root of P. lactiflora Pall (He and Dai, 2011). However, it was still not clear how these compounds differentially and commonly play a role in functional aspect. Therefore, research studies have been focused on investigating their pharmacological effects (Yoo et al., 2018), however a comparison of the anti-inflammatory effects of paeoniflorin and oxypaeoniflorin has not yet been intensively explored at various experimental conditions to deeply understand its molecular mechanism.
One of the well-known paeony’s functions is the regulation of the free radical oxidation (Qin and Tian, 2011). Free radical in the body induces aging, loosening of skin elasticity, deeper wrinkles, and cell degeneration which can lead to cancer cell formation (Lobo et al., 2010). Research has been continuously carried out to develop new antioxidant materials which can slow down skin aging and prevent the development of cancer due to free radicals (Yan et al., 2004). It is believed that polyphenol compounds, which contain a large amount of hydroxyl groups in plants can have excellent anti-oxidant effects, since they play a role in terminating radical formation by the attachment of the free radicals to the hydroxyl groups (Choudhary et al., 2008). One may wonder which components in the paeony are responsible for this antioxidant activity.
Paeoniflorin is a compound which is one of the major constituents of an herbal medicine derived from P. lactiflora, with origin of Asia (He and Dai, 2011; Suh, 2001; Wang et al., 2014; 2019; Ye et al., 2003). In Paeonia, paeoniflorin can make similar compounds with phenolic substituents (Cheng et al., 1999; Li et al., 2017; Liu et al., 2019; Takeuchi et al., 1991; Tanaka et al., 2000). In female rat study, paeoniflorin suppressed the testosterone levels in the ovaries by increasing the aromatase activity (Grant, 2012; Takeuchi et al., 1991). In mice, paeoniflorin protected against depression-like behavior induced by IFN-alpha and neuroinflammation (Li et al., 2017). Oxypaeoniflorin is homologue of paeoniflorin and can also be extracted from the roots of P. lactiflora (He and Dai, 2011). It differs from paeoniflorin since it has a p-hydroxylbenzoyl group (Furuya et al., 2012). However, a comparison of the anti-inflammatory and other related effects of paeoniflorin and oxypaeoniflorin has not yet been explored extensively (Yoo et al., 2018; Zhang et al., 2017). Therefore, in this study, we measured and compare the anti-inflammatory effects of oxypaeoniflorin with that of paeoniflorin using nitric oxide as a biomarker which measures the amount of nitrite and nitrate. Antioxidative activities of oxypaeoniflorin with that of paeoniflorin using DPPH as a biomarker which measures the amount of free radicals.
In view of the functioning of paeoniflorin (Jung et al., 2010; Lee et al., 2001; Soka, 1985), the main factors of anti-oxidative and anti-inflammatory effects of peony leaf extracts in this study can be presumed to be the function of alkaloids. To investigate this possibility, we examined the anti-oxidant and anti-inflammatory activities of paeoniflorin and oxypaeoniflorin in this study.
Materials and Methods
Cell culture
The RAW 264.7 murine macrophage cells (KCLB No. 40071) were purchased from the Korean Cell Line Bank, Korean Cell Research Foundation (Ralph and Nakoinz, 1977). They were cultured in DMEM (Dulbecco’s Modified Eagle’s Medium) supplemented with 10% fetal bovine serum, 100 U/mL penicillin, 100 ug/mL streptomycin, GlutaMAX cell culture media, and HEPES buffer (10 mM) at 37℃ in a 5% CO2 incubator. DMEM, Fetal bovine serum, penicillin, streaptomycin, GlutaMAX and HEPES were purchased from GIBCO, Thermofisher Scientific, MA, U.S.
Red Charm Extracts
Paeonia lactiflora cv. ‘Red Charm’ paeony was obtained from Woori flower, Seed and Seedling, Co. Ltd., Korea, cultivated at the University of Suwon, Korea and lyophilized as previously prepared (Kim et al., 2016). Briefly, peony flower petals were collected during May-June and dried in oven. The samples were homogenized with mixer, extracted with 70% ethanol and concentrated with Eyela evaporator (EYELA NY U.S.), and lyophilzed with freeze dryer. A concentration of 1% was used for the experiments.
Reagents
Oxypaeoniflorin and paeoniflorin were purchased from Ensol Biosciences, Inc. (Daejeon, Korea) and ALB Technology (Henderson, NV U.S.), respectively. They were dissolved in ethanol (Duksan, Korea). The 3-(4,5-methylthiazol-2-yl)-2, 5-diphenyltet-razolium bromide (MTT), DMSO and the Griess reagent were purchased from Sigma-Aldrich (St. Louis, MO, USA).
DPPH radical scavenging assay
To determine the anti-oxidation activities with DPPH (1,1-diphenyl-2-picryl hydrazyl, Sigma Chemical Co. (St. Louis, MO, USA) assay, 100 μL of paeoniflorin (600, 800, 1,000 μg/mL) and oxypaeoniflorin (600, 800, 1,000 μg/mL) was mixed with 100 μL of DPPH solution (0.2 mM) in a 96-well plate. After 30 minutes of reaction mixture (37℃), the absorbance was measured at 517 ㎚ using an ELISA reader (Epoch, Biotek Instruments, Inc. U.S.). The control solution was prepared by mixing 100 μL ascorbic acid (50 ㎎/mL) with 100 μL DPPH solution (0.2 mM). The mixture of 100 μL methanol and 100 μL DPPH solution (0.2 mM) served as blank (Sharma and Bhat, 2009).
Nitric Oxide Assay
RAW 264.7 cells were seeded in a 96-well plate at a concentration of 3.0 × 10⁵ cells/well and incubated for 24 hours. Then, the cells were treated with varying concentrations of paeoniflorin and oxypaeoniflorin (5, 50, 250, 500 μg/mL), followed by 1 μg/ml LPS (SIGMA-Aldrich, MO, U.S.) treatment. After 24 hours, in a separate 96-well plate, 100 μL of the supernatant from each well was mixed with the same amount of Griess reagent, which detects the presence of nitrite ion in solution. The absorbance was measured at 540 ㎚ using an ELISA reader (Epoch, Biotek Instruments, Inc. VT, U.S.) after 10 minutes of incubation at 37℃ (Han and Lee, 2013; Park, 2019).
MAP Kinase (ERK) phosphorylation assay
RAW 264.7 macrophage cells were seeded in a 96-well plate at a concentration of 3.0 × 104 cells/well. The cells were treated with LPS (1 μg/mL) and with the working concentrations of paeoniflorin and oxypaeoniflorin (250, 500 μg/mL) for 24 hours. The supernatant was removed and the cells were washed with PBS. MAP Kinase p42/p44 ERK ELISA kit (ab176660) was purchased from Abcam (Cambridge, U.K.) and the manufacturer’s instructions were followed. The absorbance was then measured at 450 ㎚ with an ELISA reader (Epoch, Biotek Instruments, Inc. VT, U.S.). The protein concentration of Erk antibody was measured by UV-VIS-NIR spectroscopy at the Center for Advanced Materials Analysis, The University of Suwon, Republic of Korea.
MTT cell proliferation assay
To determine the toxicity of the plant extract, RAW 264.7 cells were plated at 5 × 104 cells/ well in a 24-well plate. After 24 hours, the cells were treated with or without LPS (1 μg/mL) and with the working concentrations of paeoniflorin and oxypaeoniflorin (5, 50, 250, 500 μg/mL). Incubation was for 24 hours. For the control group, PBS (phosphate-buffered saline) was added and the cells were cultured under the same conditions. The (3-(4,5-dimethythiazol-2-yl)-2,5-diphenyl Tetrazolium MTT was dissolved in PBS with a final concentration of 5 mg/mL. Each well was treated with 200 μl of MTT solution and incubated at 37℃ in a CO2 incubator for 3.5 hours. Then, the supernatant was removed and 1 mL of DMSO was added to each well. For 15 minutes, the plate was put in the shaking incubator. Absorbance was measured at 595 nm using an ELISA reader (Carmichael et al., 1987).
Statistics
The experimental results of this study were repeated three times independently, and the results were expressed as mean ± standard deviation. Statistical analysis was performed using ANOVA (Analysis of variance) and the Student’s t-test where p values < 0.05 were considered significant.
Results and Discussion
Our previous FT-ICR MASS spectrophotometry and chemical structure data base analysis (Kim et al., 2016) identified various chemicals in the flower petal extract of peony, presumed to play a role in brightening, anti-oxidant and anti-inflammatory effects. Among these chemicals identified in this peony extract, we paid attention to oxypaeoniflorin and paeoniflorin. Paeoniflorin has been known to be a compound which is one of the major constituents of a traditional herbal medicine derived from P. lactiflora (1). Paeoniflorin inhibits the expression of testosterone in ovaries by enhancing the activity of aromatase in rat (Grant, 2012; Takeuchi et al., 1991). Paeoniflorin also protects against inflammation in brain and IFN alpha-induced depression-like behavior (Li et al., 2017).
In this study, we therefore examined that oxypaeoniflorin and paeoniflorin might play a functional role in inflammation and brightening in our RAW 264.7 macrophage cell culture system. As the first step, we investigated the effects of these molecules on viability of these cells.
Effects of paeoniflorin and oxypaeoniflorin on cell viability of RAW 264.7 macrophages
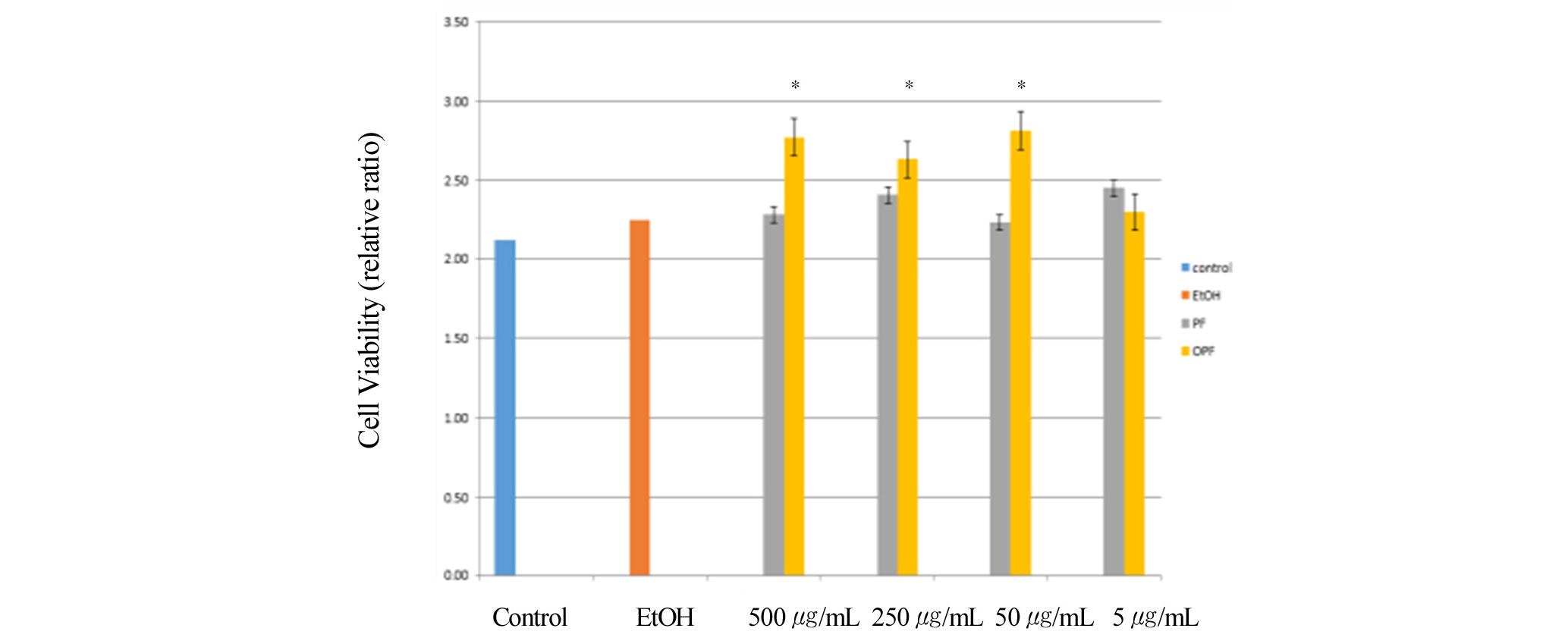
The viability of cells in response to paeoniflorin and oxypaeoniflorin was measured by MTT cell proliferation assay. After the RAW 264.7 macrophage cells were treated with or without LPS and then treated with paeoniflorin and oxypaeoniflorin, cell viability was evaluated using the MTT cell proliferation assay within the experimental concentration of paeoniflorin (PF) for 500, 250, 50, 5 ug/mL, and oxypaeoniflorin (OPF) for 500, 250, 50, 5 ug/mL indicated in Fig. 1a. Control is with PBS (phosphate-buffered saline) with 1 ug/mL LPS treatment. EtOH is treated with LPS (1 ug/mL) and ethanol. Cell viability was measured by counting viable cells after treatment. Compared to control, paeonflorin treated cells at its concentration of 500, 250, 50, 5 ug/mL showed 100-110% cell viability whereas oxypaeoniflorin-treated cells at its concentration of 500, 250, 50, 5 ug/mL indicated 100-120% cell viability. These results suggest that paeoniflorin and oxypaeoniflorin do not show any significant cytotoxic effect (Fig. 1a). To further confirm these results, we then measured absorbance at OD595 after treated MTT with Paeoniflorin and Oxypaeoniflorin treatment. Interestingly, paeoniflorin treatment with concentration of 500, 250, 50, 5 ug/mL showed 105-115% cell viability compared to control, whereas oxypaeoniflorin treatment with concentration of 500, 250, 50, 5 ug/mL indicated 110-130% cell viability compared to control (Fig. 1b). These results further suggest that paeoniflorin and oxypaeoniflorin do not show any significant cytotoxic effect in macrophages. Previous studies showed that P. lactiflora cv. ‘Red Charm’ Flower Petal Extracts might have radical scavenging activity, which shows anti- oxidant activity (Kim et al., 2016). Paeoniflorin and oxypaeoniflorin are one of the major components in Red Charm, we wondered whether paeoniflorin and oxypaeoniflorin might have effects on radical scavenging activity in macrophages.
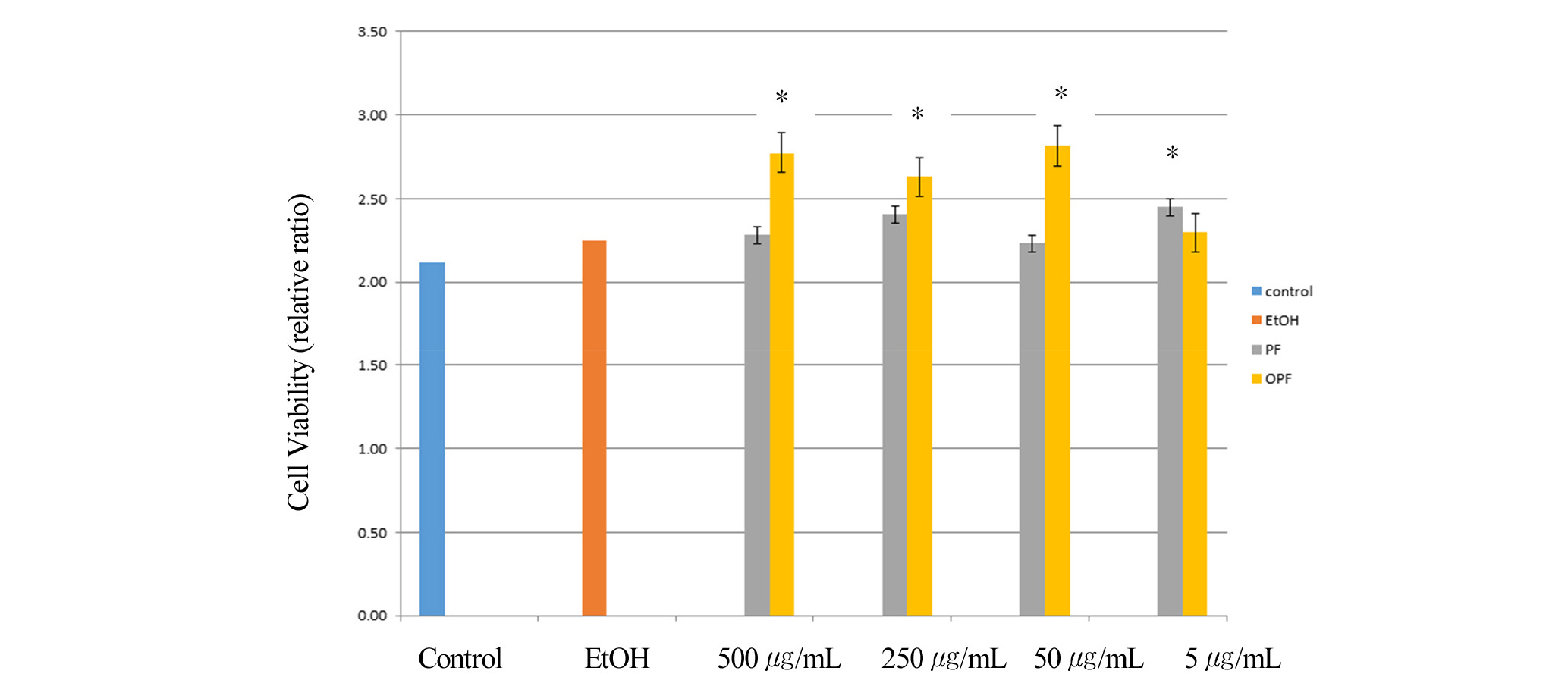
Fig. 1a.
Cells were pre-treated with paeoniflorin (5, 50, 250, 500 ㎍/mL), and oxypaeoniflorin (5, 50, 250, 500 ㎍/mL) for 1 min and then co-treated with LPS (1 ㎍/mL) for 24 h. (1a) Cell survival was indicated as % standard relative to control. Concentrations of Paeoniflorin (PF) and Oxypaeoniflorin (OPF) were used, indicated. Control is with PBS (phosphate-buffered saline) and LPS treatment. EtOH is treated with PBS, LPS (1 ㎍/mL) and 10 ul ethanol. *p < 0.05. The experiment was performed in triplicate. Error bars were calculated using Excel 2013 Formula.
Effects of oxypaeoniflorin and paeoniflorin on Radical Scavenging Activity in RAW264.7 macrophage cells
Previous studies indicated that Paeony extract has anti-oxidant activity (Kim et al., 2016). We wondered that Paeoniflorin isolated from Paeony extract might have a role in anti-oxidant effects. In the same experimental setting above with MTT assay, we first wanted to measure anti-oxidant activity of paeoniflorin and oxypaeoniflorin. Because DPPH Assay has been widely used to measure its oxidant activity (Kim et al., 2016), we employed DPPH assay to examine its anti-oxidant activity. In this study, oxypeoniflorin demonstrated 80%- 90% radical scavenging activity at its concentration of 600, 800, 1000 ug/mL, compared to ascorbic control (Fig. 2). This suggests that compared to ascorbic acid (control), the DPPH radical scavenging activity of oxypaeoniflorin had no significant differences with ascorbic control (Fig. 2). Oxypaeoniflorin showed a higher and similar radical scavenging activity compared to ascorbic acid (control) (Fig. 2), however interestingly Paeoniflorin demonstrated 5-10% radical scavenging activity itsconcentration of 600, 800, 1000 ug/mL, compared to ascorbic control (Fig. 2). This indicates paeoniflorin showed significantly reduced radical scavenging activity to compared to ascorbic control (Fig. 2). This result suggests that oxypaeoniflorin has strong anti-oxidant potential similar to ascorbic acid, but paeoniflorin shows much less anti-oxidant activity. This indicates that although structures of oxypaeoniflorin and paeoniflorin are very similar, their anti-oxidant functions are quite different selectively. This is reminiscent of the result of the HPLC-MS analysis showing that the pharmacokinetic parameters of paeoniflorin and oxypaeoniflorin were significantly different in rat experiment (Feng et al., 2010). We then explored the possibility of oxypaeoniflorin in regulating anti-inflammation effects.
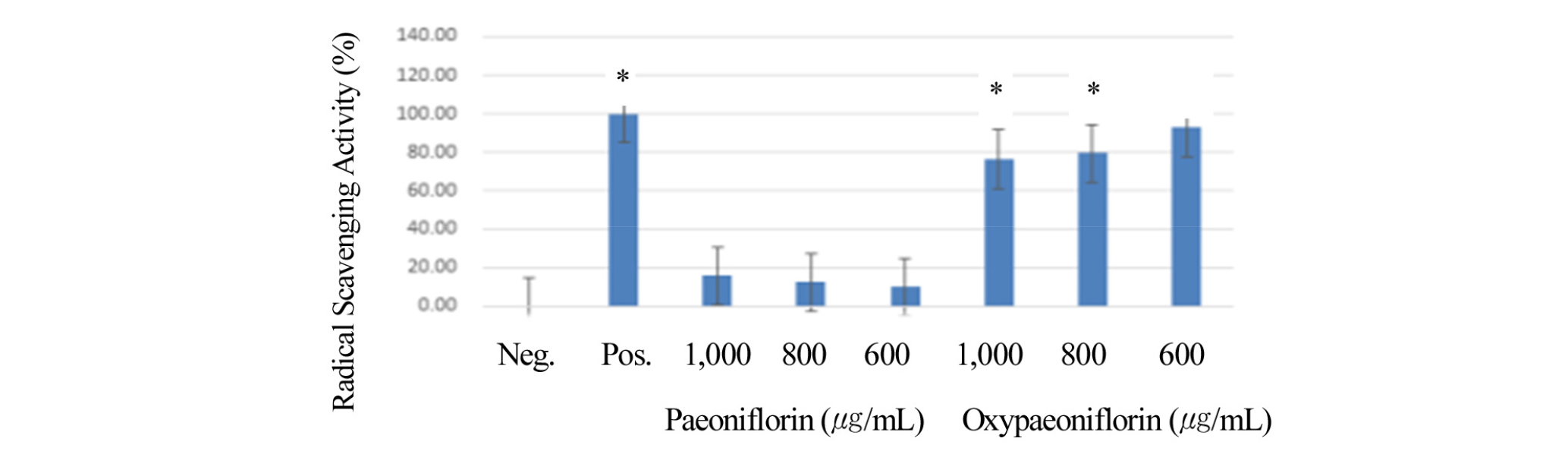
Fig. 2.
Cells were pre-treated with paeoniflorin (5, 50, 250, 500 ㎍/mL), and oxypaeoniflorin (5, 50, 250, 500 ㎍/mL) for 1 min and then co-treated with LPS (1 ㎍/mL) for 24 h. Anti-Oxidant activity was measured by DPPH assay as follows: Radical scavenging activity (relative % to control) of paeoniflorin and oxypaeoniflorin by concentration (600, 800, 1000 ㎍/mL) were indicated. Ascorbic acid is used as a positive control. Methanol solvent is used as a negative control. *p < 0.05. The experiment was performed in triplicate. Error bars were calculated using Excel 2013 Formula. X-axis Legend. 1. Negative control 2. Positive control 3. 1000 ㎍/mL Paeoniflorin 4. 800 ㎍/mL Paeoniflorin 5. 600 ㎍/mL Paeoniflorin 6. 1000 ㎍/mL Oxypaeoniflorin 7. 800 ㎍/mL Oxypaeoniflorin 8. 600 ㎍/mL Oxypaeoniflorin.
Paeoniflorin and Oxypaeoniflorin are one of the major components in Red Charm which we previously showed that it has radical scavenging activity (Kim et al., 2016). To measure Radical Scavenging Activity oxypaeoniflorin and paeoniflorin, we performed DPPH radical scavenging Assay to determine anti-oxidant activity. Compared to ascorbic acid (control), the DPPH radical scavenging activity of oxypaeoniflorin at concentration of 600, 800, 1,000 ug/mL was 80- 90% activity (Fig. 2). Paeoniflorin at concentration of 600, 800, 1000 ug/mL showed 10-20% DPPH radical scavenging activity, compared to ascorbic control. Radical scavenging activity (relative % to control) of paeoniflorin and oxypaeoniflorin by concentration (600, 800, 1,000 ug/mL) were indicated (Fig. 2). Ascorbic acid is used as a control (Fig. 2). These results suggest that oxypaeoniflorin has similar anti-oxidant potential as much as ascorbic acid whereas paeoniflorin gives significantly reduced anti-oxidant activity compared to ascorbic control. This indicates oxypaeoniflorin shows a strong anti- oxidant potential, however, paeoniflorin shows much less anti-oxidant potential. This implies that although chemical structures of paeoniflorin and oxypaeoniflorin are quite similar, their anti-oxidant potential is quite different. We can hypothesize that this study can provide the basis for further studies in the development of new anti-oxidant agents. We then ascertained whether oxypaeoniflorin might have anti- inflammatory effects.
Effect of paeoniflorin and oxypaeoniflorin on Nitric Oxide production
Lipopolysaccharide (LPS) treatment is known to cause an inflammatory response in macrophages and stimulate the production of nitric oxide (NO) (Min et al., 2010). Consequently, NO produced may lead to systemic inflammation, which may have various negative effects on the living body (Kim and Ha, 2010). In some studies, Paeoniflorin has shown to exhibit anti-inflammatory effects (Kim and Ha, 2009; 2010), however, the anti-inflammatory effects of oxypaeoniflorin, has not been explored. Therefore, we compared anti- inflammatory effects of paeoniflorin with oxypaeoniflorin. Anti-inflammatory response was determined by amount of nitric oxide (NO) production. The NO production was significantly higher in the LPS-treated group (LPS) than in the control group (LPS-untreated group). First, the control (LPS-untreated) group showed a 52% decrease in NO production, when compared to the LPS-treated cells (Fig. 3). Red Charm extract control also showed 65% decrease in NO production (Fig. 3). Paeoniflorin also showed lesser inhibition of NO production with approximately 60% decrease at 500 ug/mL compared to LPS control (Fig. 3). Paeoniflorin showed lesser inhibition of NO production with a 45% decrease at 250 ug/mL compared to LPS control (Fig. 3). Paeoniflorin also showed lesser inhibition of NO production with approximately 50% decrease at 50 ug/mL compared to LPS control (Fig. 3). Paeoniflorin also showed lesser inhibition of NO production with approximately 50% decrease at 5 μg/mL compared to LPS control (Fig. 3). In this study, Oxypaeoniflorin was highly effective in inhibiting NO production, similar to paeoniflorin (Fig. 3). When oxypaeoniflorin was compared with the LPS control, all concentrations demonstrated a significant decrease of NO production except at 5 μg/mL. The amount of NO production was indicated as a percentage relative to LPS treatment (100%) in response to Paeoniflorin and oxypaeoniflorin by concentration (Fig. 3).
Fig. 3.
Anti-inflammatory response is determined by amount of Nitric Oxide (NO) production. Cells were pre-treated with the Red Charm extract (100 ㎍/mL, 1%), paeoniflorin (5, 50, 250, 500 ㎍/mL), and oxypaeoniflorin (5, 50, 250, 500 ㎍/mL) for 1 min and then co-treated with LPS (1 ㎍/mL) for 24 h. The production of NO in LPS-induced RAW264.7 cells with the presence or absence of the Red Charm extract, paeoniflorin and oxypaeoniflorin using the Griess reagent at 540 ㎚. The NO production (%) of paeoniflorin and oxypaeoniflorin by concentration (5, 50, 250, 500 ㎍/mL) indicated. *p < 0.05. The experiment was performed in triplicate. Error bars were calculated using Excel 2013 Formula.
These results suggest that both paeoniflorin and oxypaeoniflorin may have strong anti-inflammatory activity. We were therefore curious whether paeoniflorin and oxypaeoniflorin might have common signaling mechanisms leading to the control of inflammation process.
Effect of oxypaeoniflorin and paeoniflorin on MAP Kinase phosphorylation
Previous studies indicated that MAP Kinase might be related to inflammation process (Son et al., 2011; 2013). Because we showed that paeoniflorin and paeoniflorin might have anti-inflammatory potentials in this study (Fig. 3), we wanted to investigate the possibility whether paeoniflorin and paeoniflorin might be related to MAP Kinase phosphorylation signaling. In order to measure MAP Kinase activity in response to LPS in macrophage cells, we performed pERK ELISA assay (Yoon et al., 2019). This pERK assay measures the total tyrosine phosphorylation amounts of p42/p44 ERK. These pERK assay results showed that there was increased tyrosine phosphorylation of ERK in response to paeoniflorin and oxypaeoniflorin in LPS-treated macrophage cells (Fig. 4). Specifically, Oxypaeoniflorin showed 1.7 times higher tyrosine phosphorylation of ERK (p42/p44 MAP Kinase) at its concentration of 250,500 ug/mL compared to LPS treatment alone (Fig. 4). On the other hand, paeoniflorin showed 1.3 times higher tyrosine phosphorylation of ERK at its concentration of 250, 500 ug/mL compared to LPS treatment alone, indicating that oxypaeoniflorin showed higher ERK tyrosine phosphorylation than paeoniflorin (Fig. 4). Oxypaeoniflorin also showed a little less phosphorylation compared to red charm flower petal extract (Fig. 4), which has been shown anti- inflammatory activities in a previous study (Kim et al., 2016). Red Charm extract showed 2.3 times higher tyrosine phosphorylation of ERK at its concentration of 1% (w/w) compared to LPS treatment alone. These results suggested that oxypaoniflorin significantly enhances tyrosine phosphorylation of p42/p44 ERK in LPS-induced RAW264.7 macrophage cells compared to paeoniflorin. These results imply that oxypaeoniflorin may have yet unidentified different role in tyrosine phosphorylation of MAP Kinase signaling in macrophage cells. In Fig. 4, the control is untreated group. Red Charm extract (1%) is highly effective control for increases of p-ERK. Each compounds in the Fig. 4 were less effective in 250-500 ug/mL concentration. One possibility is that oxypaeoniflorin and paeoniflorin may selectively and specifically regulates functional responses differentially in macrophage cells. Although further studies are needed to analyze the signaling process of this selectivity and specificity, these results imply that oxypaeoniflorin and paeoniflorin can be used to decipher TLR4 signaling pathway leading to inflammation process in macrophages as well as another signaling pathway leading to anti-oxidant activity.
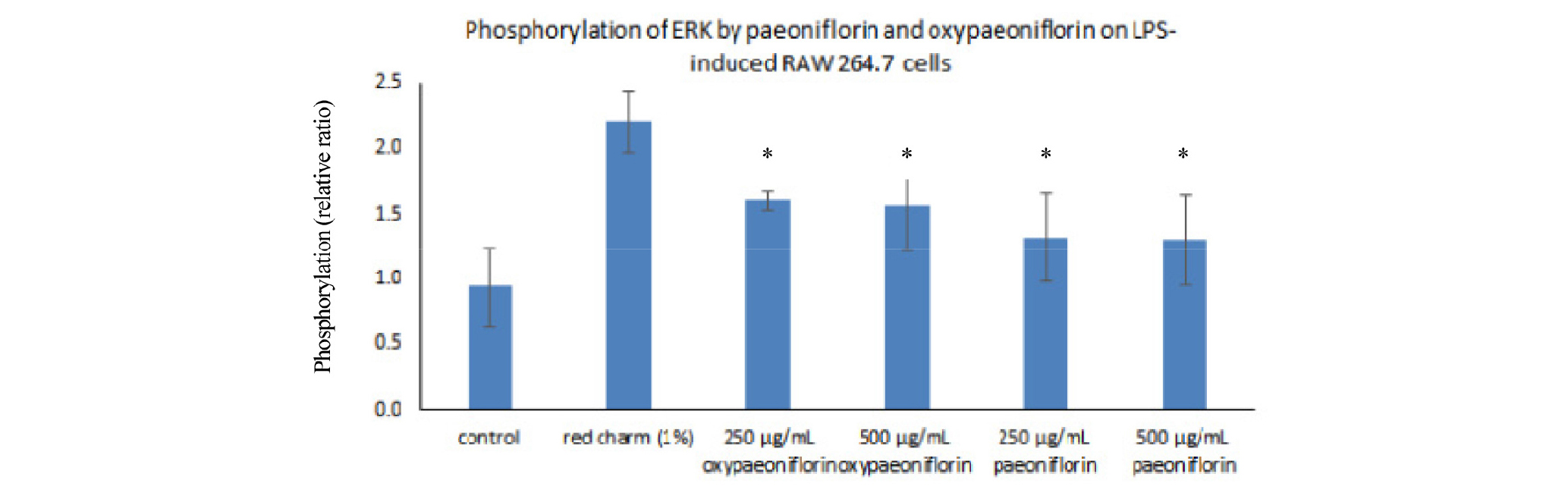
Fig. 4.
Cells were pre-treated with the Red Charm extract (100 ㎍/mL (1%)), paeoniflorin (250, 500 ㎍/mL), and oxypaeoniflorin (250, 500 ㎍/mL) for 30 seconds and then co-treated with LPS (1 ㎍/mL) for 24 h. The total *amount of tyrosine phosphorylation of ERK (p42/p44 MAP Kinase) in LPS-induced RAW264.7 cells with the presence or absence of the Red Charm extract, paeoniflorin and oxypaeoniflorin was measured by ELISA assay with the normalized equal total protein levels of macrophage cell lysates. The absorbance at 450 ㎚ in response to red charm, paeoniflorin and oxy paeoniflorin pretreatment by concentration indicated. Control is LPS-treated alone, assuming relative ratio as 1.0. *p < 0.05. The experiment was performed in triplicate. Error bars were calculated using Excel 2013 Formula.
In our study, oxypaeoniflorin reduced the level of reactive oxygen species however, oxypeoniflorin increases enhances tyrosine phosphorylation of MAP Kinase in LPS-induced macrophage. Some other group study indicated that bacteria surface–derived LPS triggers TLR signaling in macrophages, increases ROS and enhances MAPK tyrosine phosphorylation and increases inflammation (Yoo et al., 2018). However, in our study, oxypaeoniflorin increases MAPK tyrosine phosphorylation, however we showed that oxypaeoniflorin significantly reduces reactive oxygen species, leading to anti- oxidant potential, and reduces Nitric Oxide production leading to anti-inflammation potential.
Taken together, the results in this study suggest that LPS-induced inflammation is not a straightforward one-way process. Rather, oxypaeoniflorin and paeoniflorin may differentially regulate MAP Kinase signaling in LPS-treated macrophage cell culture levels and inflammation process settings. This implies that oxypaeoniflorin can be utilized as a very useful tool to decipher yet unidentified complex molecular signaling mechanisms and it can provide the basis to develop a new patient-customized therapeutics with much less side-effects.