Introduction
Material and Methods
Plant Material and Growth Conditions
Measurement of Ethylene Production
Determination of root growth and gravitropic curvature
Assay of in vitro ACC oxidase (ACO) activity
Assay of in vitro ACC Synthase (ACS) activity
Statistical Analysis
Results and Discussion
Effects of colchicine on the root growth
Effects of colchicine on the root gravitropic response
Effect of colchicine on the ethylene production in Arabidopsis root
Introduction
Plant growth and cell shapes are regulated by oriented expansion of individual cell walls. Driving force for cell expansion is osmotic, but the rate and direction of expansion are controlled by mechanical properties of the cell wall, which is characterized by both microtubules and cellulose microfibrils (Szymanski and Cosgrove, 2009).
Microtubules affect the arrangement of cellulose microfibrils, composing the plant cell wall and regulating the plant cell growth. There are several studies about the arrangement between microtubules and cellulose microfibrils to control the cell shape or tissue expansion. Emons et al. (1990) found that both microtubules and cellulose microfibrils arranged in all directions in the expanding part of the root hair. The parallelism between microtubule and cellulose microfibrils have been reported in cotton seed hairs (Seagull, 1986) and shoot apical meristem of Vinca major (Sassen and Wolters- Arts, 1992). And Baskin et al. (1999) reported that the region, where slow growth happened or no more growth showed the oblique orientation between microtubules and cellulose microfibrils, not parallel. Baskin et al. (2004) reported that cortical microtubule controlled the expansion of the Arabidopsis root and the microtubules regulated the alignment of cellulose microfibrils across the root, which made the uniform mechanical structure. In Arabidopsis hypocotyls, the alignment of the microtubule and cellulose microfibrils in outer epidermal cell wall regulated growth rate, whereas inner epidermal cell wall controlled growth direction (Chan, 2012).
Colchicine, an alkaloid produced in Colchicum autumnale, can adhere to tubulin dimers and prevent the formation of microtubule (Planchais et al., 2000; Ma et al., 2018). There was a report that colchicine inhibited the elongation of hypocotyl segments by disrupting the microtubule assembly (Durnam and Jones, 1982).
Microtubule, one of the cytoskeletons, plays important roles in the development of plant such as cell wall formation, and amyloplast sedimentation in columella cell of root tip (Hou et al., 2003). The sedimentation of amyloplast in columella cell in root tip is correlated with the sensing of gravity, resulting in the downward growth into the soil in order to absorb water and minerals (Blancaflor and Masson, 2003). The orientation of microtubule is changed depending on the level of ethylene in plant cells (Zarembinski and Theologis, 1994).
Ethylene, as a gaseous plant hormone, is involved in multiple cellular processes such as stress response, development and differentiation (Bleecker and Kende, 2000). Ethylene synthesis begins from methionine via two major intermediates, S-adenosylmethionine (AdoMet) and 1-aminocycopropane-1-carboxylic acid (ACC). ACC synthase (ACS) and ACC oxidase (ACO) catalyze the conversion from AdoMet to ACC and to ethylene, respectively. The activity of the enzyme is regulated by several factors. Auxin stimulates the production of ethylene by increasing the expression level of the ACS gene (Bleecker and Kende, 2000). There are findings that ethylene regulates root growth and gravitropism via alteration of auxin transport (Kim et al., 2000; Ruzicka et al., 2007), and ethylene and auxin are required for root to penetrate in soil (Santisree et al., 2012).
According to Schwuchow and Sack (1994), amiprophos- methyl and cytochalasin D, inhibitors of plant microtubule polymerization, increased the plastid sedimentation in the protonema of Ceratodon. Blancaflor and Masson (2003), suggesting that the sedimentation of amyloplast is a key element in sensing the gravity, and that the polymerization of microtubule affects the gravitropism. Also, ethylene can inhibit the growth of root and its gravitropism. However, there have been little evidences showing that inhibitory effect of colchicine on the root growth and its response to gravitropism is correlated with ethylene production in root. Therefore, we investigated a possibility that inhibition of microtubule assembly by colchicine and the consequential retardation of root growth and the disruption of gravitropism could be mediated via ethylene production.
Material and Methods
Plant Material and Growth Conditions
Seeds of Landsberg erecta (Ler), Arabidopsis thaliana were soaked in 70% ethanol for 5 min and washed three times with autoclaved distilled water. The sterilized seeds were planted on the solid agar (1%) medium containing half-strength of MS salts supplemented with 1% sucrose and 1 mM MES (pH 5.8). The seeds were incubated in vertical position at 4℃ for 1 day and then were incubated for another 6 days at 22℃.
Measurement of Ethylene Production
Ethylene production was measured in 100 root segments (10 ㎜) of Arabidopsis. The root segments were placed in silicon-capped vials containing 1 ㎖ of MES buffer (100 mM, pH 6.8, 50 ㎍/㎖ chloramphenicol) with the test compounds. The vials were incubated in dark at 27℃ in a shaking incubator. After incubation, 1-㎖ of gas sample was withdrawn from the vial using syringe and injected to a gas chromatograph (HP5890 Series II; Hewlett-Packard, USA) equipped with an alumina column (80/100 Porapak-Q; 1.8-m x 2.1-㎜).
Determination of root growth and gravitropic curvature
The 6-day old seedlings were placed vertical or horizontal in small petri dishes. Growth and gravitropic curvature were measured using a camera (Rexsa, DS-400 PC-camera) with the time-interval software (SupervisionCam ver. 3.2.2.4; http://supervisioncam.com). Images were recorded every 15 min and analyzed using UTHSCSA Image Tool Program (ver. 3.0; http://comdent.uthscsa.edu/dig/itdes.html).
Assay of in vitro ACC oxidase (ACO) activity
in vitro analysis of ACO activity was performed as previously described by Mekhedov and Kende (1996). One hundred of root segments were treated with 10-7 M and 10-5 M colchicine for 4 hr, and the samples were flash-frozen in liquid nitrogen. The frozen samples were ground in liquid nitrogen, and combined with extraction buffer (100 mM MES, pH 7.5, 10% glycerol, 30 mM ascorbate and 2 mM DTT). The resuspended samples were centrifuged at 15,000 rpm for 10 min at 4℃. The supernatant was transferred to a new vial containing incubation buffer (50 mM MES, pH 7.5, 10% glycerol, 30 mm ascorbate, 2 mM DTT, 30 mM NaHCO3, 50 μM FeSO4, 1 mM ACC) and incubated on a shaker for 1 hr at 22℃ in dark. After incubation, 1-㎖ of gas sample was withdrawn from the vial using syringe and injected to the gas chromatograph. The amount of ethylene production is regarded as in vitro ACO activity.
Assay of in vitro ACC Synthase (ACS) activity
ACS activity was determined according to the modified method by Woeste et al. (1999). One hundred of root segments were treated with 10-7 M and 10-5 M colchicine for 4 hr, and root segments were flash-frozen in liquid nitrogen. The frozen samples were ground in liquid nitrogen, and combined with 250 mM potassium phosphate buffer (pH 8.0) containing 10 μM pyridoxal phosphate, 1 mM EDTA, 2 mM PMSF and 5 mM DTT. Samples were centrifuged at 15,000 rpm for 15 min at 4℃. The supernatant was incubated with 5 mM AdoMet (0.1 ㎖) for 1 hr at 22℃. Then ACS activity was measured by adding a mixture of 0.1 ㎖ of 20 mm HgCl2 and 0.1 ㎖ of NaOH/NaOCl (saturated NaOH : 5% NaOCl = 1 : 1 [v/v]) to the supernatant to produce ethylene, and the reaction was stopped by incubating the solution on ice for 10 min. The ethylene production was measured as described above.
Statistical Analysis
All experiments were conducted at least three times, with no fewer than 40 primary roots each. To test for significance at p values of <0.05, the data mean values were calculated according to two-way ANOVA test and Duncan test.
Results and Discussion
Effects of colchicine on the root growth
The growth of Arabidopsis root was inhibited by the treatment of colchicine (Fig. 1). The inhibition of root growth was observed within 2 hr after colchicine treatment at a concentration of 10-7 M and 10-5 M and the inhibition was maintained for 8 hr, and the inhibition rates of root growth were about 25% and 40% when compared to control, respectively.
It has been known that cortical microtubules control expansion of the Arabidopsis root, and regulate alignment of microfibrils across the root (Baskin et al., 2004). Colchicine is a cytoskeletal disrupting agent, whose treatment showed a reduction in the number of microtubules in developing cotton fibers (Seagull, 1990). Cytoskeletal elements, in particular microtubules, play a role in regulating cell morphogenesis by determining the orientation and location of cellulose microfibril deposition (Yatsu and Jacks, 1981). Therefore, the result of Fig. 1 suggested that the inhibition of root growth in the elongation zone can be attributed to the disruption of microtubule assembly caused by the colchicine.
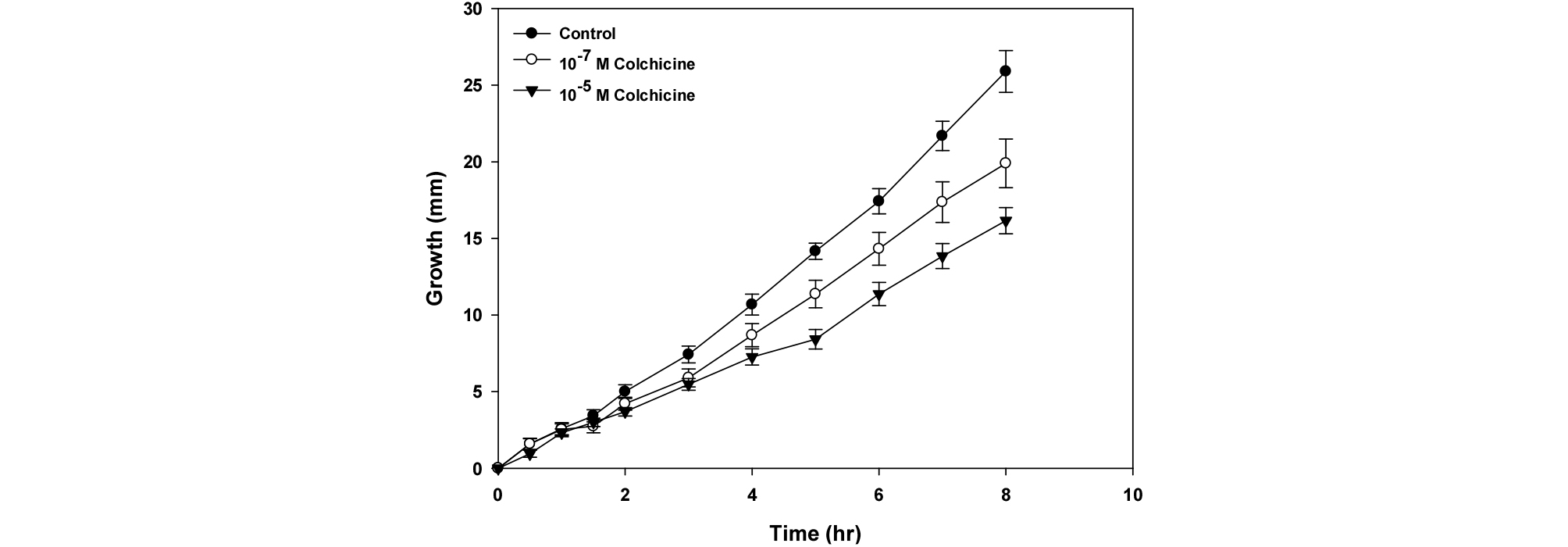
Fig. 1.
Effect of colchicine on root growth in Arabidopsis seedlings for 8 hr. Colchicine was applied to the root using an agar plate. After a vernalization for 1 day at 4℃, seeds were grown for 6 days under diurnal rhythm such as day and night was 16 hr and 8 hr, respectively. These seedlings were transferred the agar plate containing several concentrations of colchicine such as 10-7 M and 10-5 M, and placed in vertical position. The growth was measured for 8 hr using a camera as described in Material and Methods. Symbols are mean values ± SE from 10 independent experiments.
Effects of colchicine on the root gravitropic response
When vertically grown seedlings are positioned perpendicular, roots with no treatment of colchicine started showing gravitropic response within 15 min and the curvature reached almost 90° at 8 hr after gravitational treatment (Fig. 2). The roots treated with colchicine at a concentration of 10-7 M showed a similar pattern to control in the course of 3 hr treatment, however, the gravitropic response decreased 8 hr after treatment. At higher concentration of colchicine 10-5 M, root curvature reached 60° 2 hr after treatment, which showed 30% reduction of gravitropic response compared to control. After 4 hr, roots showed similar pattern of gravitropic curvature regardless of colchicine concentrations. This data suggest that higher concentration of colchicine delayed gravitropic response but at the lower concentration of colchicine, the gravitropic response was not delayed, but it can inhibit gravitropic response from the point of 4 hr treatment to 8 hr.
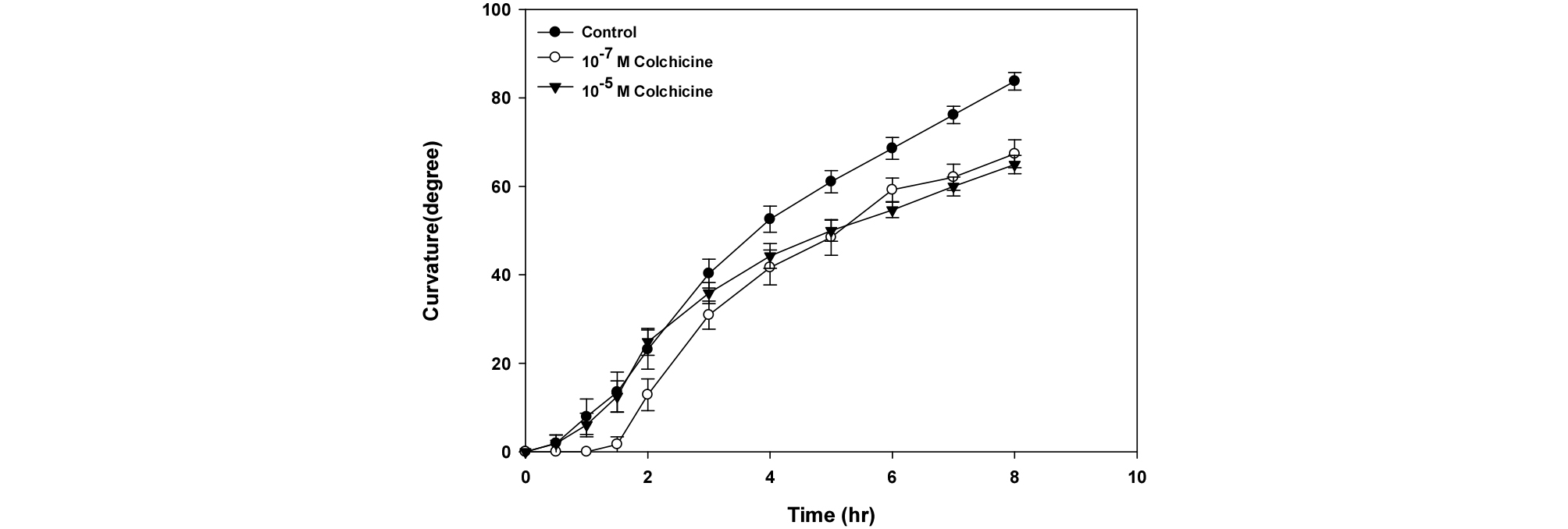
Fig. 2.
Effect of colchicine on the gravitropic response in Arabidopsis seedlings for 8 hr. After a vernalization for 1 day at 4℃, seeds were grown for 6 days under diurnal rhythm such as day and night was 16 hr and 8 hr, respectively. These seedlings were transferred the agar plate containing several concentrations of colchicine such as 10-7 M and 10-5 M, and placed in horizontal position. The growth was measured for 8 hr using a camera as described in Material and Methods. Symbols are mean values ± SE from 10 independent experiments.
Gravitropic response in roots results from differential growth between upper and lower side of roots in horizontal position. Gravitropism in roots could be occurred via 3 steps; (i) the perception of gravity in root tip, (ii) signal transduction form root tip to the elongation zone and (iii) response in elongation zone to make curvature (Chen et al., 1999). Microtubule is involved in the sedimentation of amyloplast in columella cell of root tip (Hou et al., 2003). It has been known that the perception of gravity is associated with the movement of amyloplast in columella cell of root tip. The movement of amyloplast might initiate physiological changes in the root tip cells via the interaction between the amyloplast and other cellular component such as endoplasmic reticulum, or cytoskeletal elements. Therefore, the delayed gravitropic response by the treatment of colchicine might be due to the interruption of amyloplast sedimentation because of the disruption of microtubule alignment in root tip or in root elongation zone.
There have been several reports that ethylene affects growth and gravitropism in plants. Lee et al. (1990) suggested that ethylene may modify positive curvature in the primary roots of maize by affecting gravity induced-lateral auxin transport. Kim et al. (2000) presented that an optimal concentration of ethylene might be required for the regulation of gravitropism in maize roots. In addition, there are other studies showing a relationship between ethylene and microtubule alignment. Yuan et al. (1994) reported that cortical microtubule reoriented in wounding pea stem from transverse to vertical orientation. As well known, wounding in plants cause to produce ethylene production to protect them (Bleecker and Kende, 2000). Therefore, we measured change of ethylene production under the treatment of colchicine in Arabidopsis root.
Effect of colchicine on the ethylene production in Arabidopsis root
External application of colchicine stimulated the ethylene production at the concentration of 10-7 M and 10-5 M for 8 hr treatment (Table 1). At the concentration of 10-5 M, colchicine increased the ethylene production 38% over control at 2 hr. Ethylene affects the growth pattern of plants by reducing the rate of elongation and increasing lateral expansion, leading to swelling of the tissue and modifying the gravitropic response (Bleecker and Kende, 2000). Thus, the inhibition of growth and curvature by colchicine might be due to the reorientation or disruption of microtubule in the root tip or in the elongation zone. Ruzicka et al. (2007) reported that ethylene stimulated auxin biosynthesis and increased the capacity of auxin transport by regulating the transcription of auxin transport components such as AUX1 and PIN2. The increase in auxin production by ethylene could alter distribution of auxin from root cap to the elongation zone via outer cell layer, and auxin could inhibit growth in the elongation zone. Recently, Ma and Ren (2012) reported during the germination of flax, the gravitropic response of the flax root become weaker due to the decrease in auxin sensitivity and transport, which was caused by increased ethylene production. Therefore, possible increase of ethylene production by colchicine could affect auxin movement and/or lateral auxin transport in horizontal roots. Ma et al. (2017) reported that ethylene regulates root elongation and its gravitropic responses via the alignment of microtubule.
Table 1. Effect of colchicine on ethylene production in root segments of Arabidopsis for 8 hr. After a vernalization for 1 day at 4℃, seeds were grown for 6 days under diurnal rhythm such as day and night was 16 hr and 8 hr, respectively. Root segments (10 ㎜) were incubated for 8 hr in solution containing 10-7 M and 10-5 M colchicine. At every 2 hr, 1 ㎖ of gas sample was withdrawn from the vials for measuring ethylene production
| Colchicine (M) | Ethylene production (nl/100 segments) | |||
| 2 hr | 4 hr | 6 hr | 8 hr | |
| 0 | 32.7±3.9 | 51.5±3.6 | 74.5±4.0 | 102.1±3.6 |
| 10-7 | 38.9±1.8 | 58.1±2.1 | 77.3±3.9 | 112.4±2.1 |
| 10-5 | 45.4±3.3 | 63.6±2.9 | 91.4±3.0 | 117.0±2.9 |
We examined the possibility that the observed inhibitory effect on the root growth and the disruption of curvature by the treatment of colchicine could be mediated via increased ethylene production. The inhibition of the growth by colchicine was recovered by the addition of 10-4 M cobalt ion, which is an inhibitor of ethylene production (Yu and Yang, 1979). In Table 2, cobalt ion stimulated root growth 80% over control at 8 hr, and recovered the 10-5 M colchicine-induced inhibition of growth. This data means that colchicine inhibited the root growth via the stimulation of ethylene production.
Table 2. Effect of colchicine on root growth in Arabidopsis seedlings for 4 hr and 8 hr. After a vernalization for 1 day at 4℃, seeds were grown for 6 days under diurnal rhythm such as day and night was 16 hr and 8 hr, respectively. These seedlings were transferred the agar plate containing 10-4 M cobalt ion and/or 10-5 M colchicine, and placed in vertical position. The growth was measured for 4 hr and 8 hr using a camera as described in Material and Methods
| Treatment | Root growth (㎜) | |
| 4 hr | 8 hr | |
| 0 | 6.5±0.5 | 12.5±0.8 |
| Co2+ | 6.3±0.3 | 16.0±0.5 |
| Colchicine | 6.1±0.6 | 9.1±0.6 |
| Colchicine + Co2+ | 7.1±0.6 | 13.5±0.7 |
To confirm the effect of colchicine on ethylene production, we measured in vitro activities of ACC synthase (ACS) and ACC oxidase (ACO). In vitro ACO and ACS activities were increased 20%~50%, depending on the colchicine concentrations 4 hr after treatment (Fig. 3). Therefore the increased production of ethylene by colchicine was mediated via the activation of ACS and ACO.
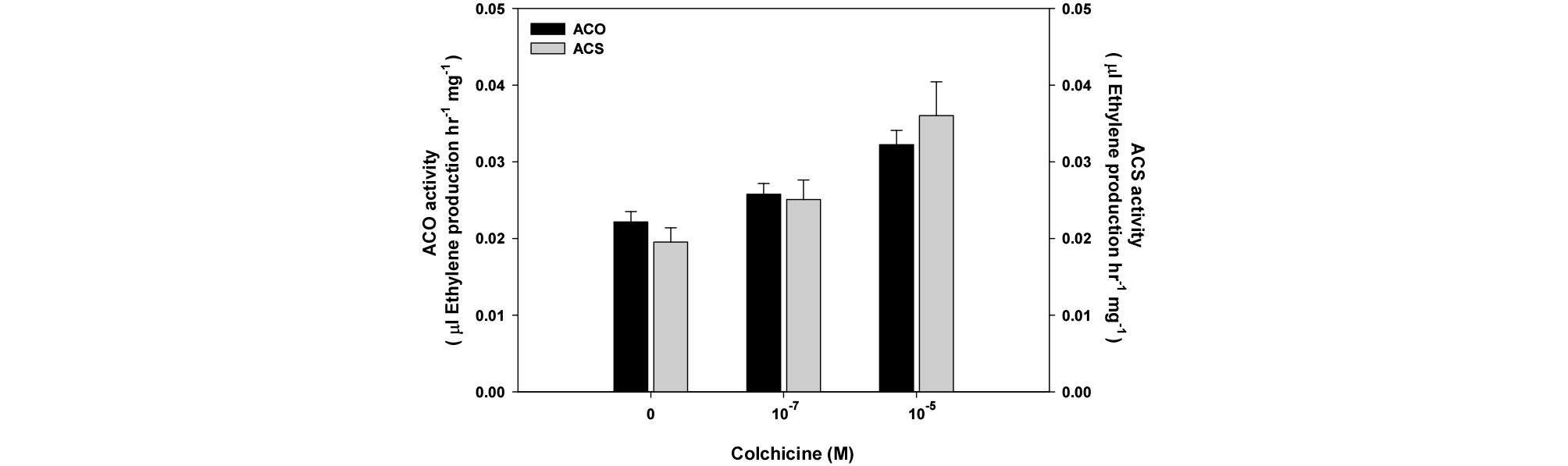
Fig. 3.
Effect of colchicine on in vitro ACS and in vitro ACO activities. To measure in vitro ACS activity, one hundred of roots were incubated for 4 hr in solution containing 10-7 M and 10-5 M colchicine. These root segments were ground in liquid nitrogen, and added to 250 mM phosphate buffer, and ethylene production was measured as described in Material and Methods. To measure in vitro ACO activity, one hundred of roots were incubated with colchicine for 4 hr. These root segments were ground in liquid nitrogen, and added to an extraction buffer. After centrifugation, the supernatant was transferred to a new vial containing incubation buffer. Ethylene production was measured as described in Material and Methods to detect ACO activity. Symbols are mean values ± SE from 10 independent experiments.
In conclusion, the inhibition of root growth and its reduced gravitropic response in Arabidopsis by the treatment of colchicine was occurred via the stimulation of ethylene biosynthesis, which was attributed to the stimulated ACS and ACO activities.




