Introduction
Materials and Methods
Plant material and growth condition
Treatment of ethephon and investigation of growth characteristics on plants and seeds
Statistical analysis
Results and Discussion
Introduction
Hemp (Cannabis sativa L.) originated from Central Asia and has been cultivated for fiber production for thousands of years in Korea (Moon et al., 2006). Hemp seeds have been used for the production of functional food and cosmetics in Europe and North America because of its rich non-saturated fatty acids, which include γ-linolenic acid (Moon et al., 2005). Hemp belongs to the family Cannabaceae and an anemophilous plant that is pollinated by wind (Cabezudo et al., 1997; McPartland, 2018; Small, 2015).
There are some debates about the speciation of hemp regarding whether they are divided into two species (C. sativa and C. indica) or one species (Clarke and Merlin, 2016). However, most researchers believe that C. indica and C. sativa are one species because there are no barriers for hybridization between the species by pollination (Beutler and Marderosian, 1978; de Meijer and Van Soest, 1992; McPartland, 2018).
Recently, hemp has received attention for medicinal use with cannabinoids produced in fertilized female flowers (Chandra et al., 2017). The cannabinoids are synthesized at glandular trichomes which are abundant on bracts of female flowers (Clarke and Merlin, 2016; Kim, 2019). The cannabinoid content of bracts were highest in maturing stage but decreased with being senescent (Mahlberg and Kim, 2004; Zager et al., 2019). The seed borne bracts were early senescent with maturing seeds whereas non-seeded bracts were late senescent (Clarke and Merlin, 2016). Thus, for the production of medicinal cannabinoids, female plants of dioecious cultivars should be cultivated exclusively to prevent fertilization with pollen from male flowers of male or monoecious plants (Chandra et al., 2017; Spitzer-Rimon et al., 2019).
Separate sexes were evolved from hermaphroditic ancester having both stamens and carpels in the same flower in plantae (Aryal and Ming, 2014; Bai et al., 2019; Moliterni et al., 2004). Although there are some monoecious cultivars that produce male and female flowers on the same plant, most of hemp cultivars are dioecious that produces male (pollen) flowers in male plants and female (seed) flowers in females (Clarke and Merlin, 2016; de Meijer and Van Soest, 1992; Mandolino et al., 1999). The gender of dioecious plants is regulated by genetic, epigenetic and hormonal factor (Bai et al., 2019; Heikrujam et al., 2015). Hemp have nine pair of autosomes and one pair of sexual chromosomes which are X and Y (Moliterni et al., 2004). Male plants of hemp are heterogametic (XY) whereas females are homogametic in sexual chromosome pairs (Heikrujam et al., 2015; Moliterni et al., 2004). The sex expression of dioecious plants can be changed by the plant hormones, and ethylene is a feminizing hormone in the plantae (Heikrujam et al., 2015; Sakthinathan et al., 2017). Thus, hormone application can induce partial sexual change in dioecious plants (Aryal and Ming, 2014; Heikrujam et al., 2015).
Some breeders bred medicinal cultivars that have a large content of cannabidiol, the major medicinal cannabinoid. However, most of the cultivars were not inbred lines, but F1 or selected lines from unknown parents and therefore the cultivars should be propagated not by seeds but by vegetative propagation (Carter, 2017; Cohen, 2014, 2020; Holmes, 2019; Lewis, 2020). The effective methods to breed inbred lines are self-fertilization because selected genes are more likely to be represented in both the male pollen and the female ovules if they come from the same plant (Clarke and Merlin, 2016). Moon et al. (2010) developed a production method for feminized seeds by inducing the formation of male flowers and pollen on female plants that can be used for self fertilization of hemp. However, feminized seeds are not suitable for the production of non-fertilized flowers because the greater the generation of feminized seeds, the higher the ratio of monoecious plants (Faux et al., 2014). If male plants can produce male and female flowers on the same plant, not only can desirable traits be fixed in an early generation, but also dioeciousness can be maintained. This experiment was conducted to develop a self-fertilization method by the formation of sufficient amounts of female flowers and seeds from male plants of dioecious hemp by the timing of ethephon treatment.
Materials and Methods
Plant material and growth condition
Hemp seeds of the Korean landrace, a dioecious cultivar, were sown in a tray pot with 72 cells, and the seedlings were planted in Wagner pots (size: 1/2,000 a), 15 days after sowing. The Wagner pots were filled with nursery media with constituents of 4%, 7%, 68%, 14.7%, 0.2%, and 0.06% of zeolite, perlite, coconut peat, peat moss, fertilizer, and wetting agent, respectively. The plants were grown in Walk In Chamber with a size of 3,150 ㎜, 5,800 ㎜, and 3,050 ㎜ in width, length, and height, respectively. The environment conditions of the Walk In Chamber were adjusted to 25℃, 65%, 144 μ㏖ m-2 s-1, and 500 ㎎ ㎏-1 in temperature, humidity, luminous intensity, and concentrations of CO2, respectively. To induce early flowering, the Walk In Chamber was adjusted to a short day length with light and dark periods of 12 hours. Twenty days after planting, the sex of hemp plants was identified by the appearance of primordia at the node, and male plants were used as the material for the experiment. Ten female plants of the same cultivar were grown as controls.
Treatment of ethephon and investigation of growth characteristics on plants and seeds
The sex of hemp plants cannot be distinguished in the vegetative stage. In conditions of short day length, the plants initiate sex differentiation with the formation of primordia (Mediavilla et al., 1998; Moliterni et al., 2004). Male primordia can be identified by their curved claw shape, soon followed by the differentiation of round pointed flower buds as shown in Fig. 1 (Mediavilla et al., 1998). Sakthinathan et al. (2017) showed that spraying 500 ㎎ L-1 to Cucurbita maxima was effective to increase the number of female flowers per plant without any side effect including decrease of seed germination. Thus, ethephon 39% (NongHyup Chemical, Seongnam, Korea) was diluted to 500 ㎎ L-1 with water. The ethephon solution was sprayed onto plants at soon after primordia formation, and subsequently, seven and fourteen days later (Fig. 1).

Fig. 1
Male plants at the time of ethephon treatment after male primordia formation, soon after (A), 7 days later (B) and 14 days later (C). At the time of primordia formation, primordia were formed between stipule and petiole under shoot tip. At time goes on, leaves became smaller, decreased and more flowers were formed.
The plant ratio of female flower formation and growth characteristics, including stem height, were investigated 15 and 45 days after treatment with ethephon solution. The characteristics of seeds and germination rate were investigated using harvested seeds. The plant ratio of female flower formation was measured with ten plants per replicate. Of the ten plants, we counted the number of plants that formed stigma and calyx, and calculated the percentage. Stem height was measured from the distal end to the apex. The number of branches was counted from the first branch attached to the trunk. The number of fruiting nodes on the branch was counted as seed bearing nodes on the longest branch.
The characteristics of seeds including the number of harvested seeds were investigated after harvest. The number of harvested seeds, weight of 1,000 grains, seed size, and germination rate were measured with seeds from 6 plants in each treatment. Individual plants were regarded as replications for statistical analysis. The size, length, and width of the seeds were measured by copying the seeds with a document copier (FX DocuCentre-III 3007 PCL 6, Tokyo/Japan, Fuji Xerox) and measuring the photocopied 20 seeds per replication (Moon et al., 2020).
Statistical analysis
Duncan multiple range test was used to determine significant differences between the timing of ethephon treatment and control. Statistical analyses were carried out using the SAS statistical package (version 9.3-2012; SAS Institute, Cary, USA).
Results and Discussion
By treatment of ethephon to male plants of hemp, female flowers were formed on the shoot and branch even if the flowering amounts were different by treatment timing. The plant ratio of female flower formation was 100% in the ethephon treatment at soon after primordia formation, which was the same as that in the control of normal female plants, but decreased to 40% and 0%, at seven and fourteen days after primordia formation, respectively (Table 1). Ethephon is converted into enthylene upon metabolism by plants and exerts a potency of plant growth regulator (Potter et al., 1990; Sakthinathan et al., 2017). Ethylene has a feminization effect on the sex expression of hemp (Galoch, 1978; Moliterni et al., 2004). As the treatment of ethephon were undertaken earlier, the effect of female flower formation was greater. Stem height showed statistical significance at the 5% level among the treatment and control groups as 141㎝, 158 ㎝, 178 ㎝ and 122 ㎝ in ethephon treatment at soon, seven, fourteen days after primordia formation and control of normal female plants, respectively. However, the number of branches did not show statistical significance at the 5% level among the treatment and control groups at 26, 24, 26, and 25 in ethephon treatment at soon, seven and fourteen days after primordia formation, and control of normal female plants, respectively (Table 1). Ethylene not only accelerates the ripeness of grains or fruits but also the growth inhibition of plants (Aryal and Ming, 2014; Dubois et al., 2018; Scott and Leopold, 1967). In this experiment, the shorter stem height in the treatment of ethephon was thought to result from the effect of growth inhibition in the hemp plants.
Table 1.
Plants ratio of female flowers formation and growth characteristics by timing of ethephon treatment on male hemp plants
Female and male flowers were formed side by side on male plants which were treated by ethephon whereas normal female and male plant formed female and male flowers exclusively, respectively (Fig. 2). Male inflorescence of hemp are hanged to panicle sometimes branched, and female inflorescence are raceme developing densely at the apex of the plant or at the axils of leaves or lateral branches (Moliterni et al., 2004). In this experiment, female and male flowers were hanged to panicle sometimes branched like a normal male inflorescence.
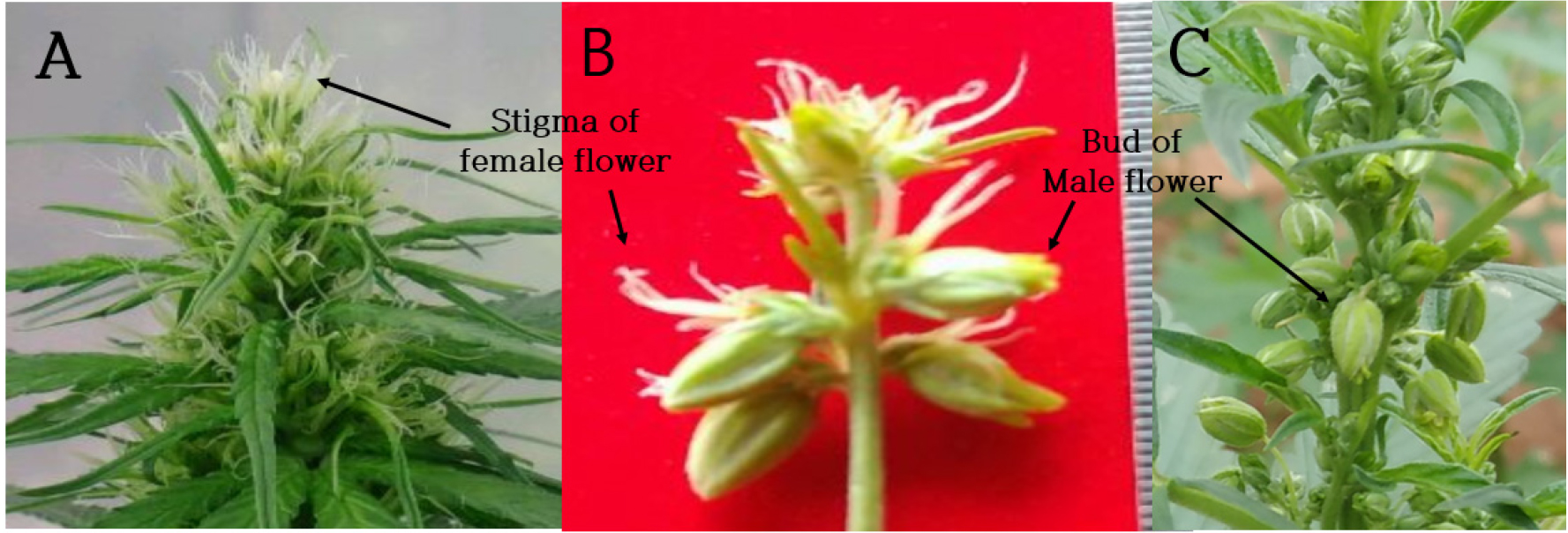
In a review by Hall et al. (2012) on inflorescence of hemp, female flowers consisted of a single ovary, bract, and dual stigmas. However, female flowers of male plants had 5 stigmas in contrast to the dual stigma of the flowers of female plants in this experiment (Fig. 3).
In male plants which were treated by ethephon, smaller seeds were formed loosely on the node, whereas female plants formed larger seeds densely (Fig. 3). The number of fruiting nodes on the branches were 17, 9, 0, and 24 in ethephon treatment at soon, seven, fourteen days after primordia formation and control of normal female plants, respectively, which were statistically significant at the 5% level (Table 2). Number of harvested seeds were also showed statistical significance at 5% level among the treatments and control as 960, 183, 0 and 1,044 in ethephon treatment at soon, seven, fourteen days after primordia formation and control of normal female plants, respectively (Table 2). As mentioned above, ethylene has a feminization effect on the sex expression of hemp. Plant hormones, including ethylene, have more effects when treated at the early growth stage (Bandara et al., 1998). As the treatment of ethephon was undertaken earlier, the number of fruiting nodes on the branch was greater. Further, with a greater number of fruiting nodes, more seeds were harvested in this experiment.
Table 2.
Fruiting characteristics of hemp plants by timing of ethephon treatment on male hemp plants
In the characteristics of harvested seeds, weight of 1,000 grains were 7.3 g, 5.1 g and 20.6 g in the ethephon treatment at soon, seven, fourteen days after primordia formation and control of normal female plants, respectively. Seed size, examined by length and width, showed statistical difference at 5% level among the treatments and control as 3.34 and 2.67 ㎜, 2.89 and 2.27 ㎜, 4.27 ㎜ and 3.36 ㎜ in ethephon treatment at soon, seven, fourteen days after primordia formation and control of normal female plants, respectively. In this experiment, the seeds from male plants were much smaller than normal seeds, which were harvested from female plants (Fig. 4). The seeds from male plants could be germinated and grown to seedlings, although the germination rate was lower than that of seeds from female plants. Germination rates were statistically different at the 5% level between seeds from male and female plants as 53–54% and 80% in seeds from male and female plants, respectively, but there was no statistically significant difference between the timing of ethephon treatment (Table 3). In conclusion, treating ethephon to male plants at soon after primordia formation will be good strategy in breeding program to breed inbred lines of hemp by self-fertilization.