Introduction
Materials and Methods
Preparation of L. lancifolium extract
Cell culture
Animals
MIA-induced osteoarthritis
Hind paw weight-bearing distribution
Blood analysis and measurement of cytokine levels
Histopathological examination
Statistical analysis
Results
LL downregulated the level of pro-inflammatory cytokines in IL-1β-induced HTB-94 cells
Oral administration of LL ameliorated the hind paw weight-bearing distribution in the rat model of MIA-induced OA
Administration of LL alleviated the level of inflammatory mediators in MIA-induced rats
LL alleviated the invasion of inflammatory cells in the joints of MIA-induced rats
Discussion
Introduction
Osteoarthritis (OA) is a progressive degenerative joint disease that accompanies cartilage erosion, synovial inflammation, subchondral bone sclerosis, and osteophyte formation (Philippe et al., 1997; So et al., 2011); the prevalence of OA has been growing with the increase in elderly population (Wojdasiewicz et al., 2014). Currently, there is no specific drug that can target the proven mechanisms of action. Nonsteroidal anti-inflammatory drugs (NSAIDs) are commonly prescribed for patients with OA, in order to relieve pain and improve the joint environment. However, long-term use of NSAIDs can cause side effects, such as stomach irritation and ulcers, and increase the risk of cardiovascular diseases (Marcum and Hanlon, 2010). According to Park et al. (2019), the use of NSAIDs in Korean patients with OA is much higher than that in the US and Spain. Therefore, treatment of osteoarthritis has been increasingly demanding the use of functional foods, in order to avoid problems related to long-term use of drugs and various other side effects.
Lilium lancifolium (LL), a medicinal herb, is a perennial plant that grows in the mountains and fields in different parts of Korea. The plant is approximately 1.5 m long, with round scales 5-8 ㎝in diameter (Park et al., 2014). LL is known to exert anti-inflammatory effects by lowering inflammatory cytokines (Kwon et al., 2010; Lee et al., 2013) and foraging activities against free radicals (Xu et al., 2016). Accumulating evidence has shown LL to regulate inflammation; however, its effect on OA has not been reported yet.
In this study, we explored the anti-inflammatory effect of LL on human chondrosarcoma cell line HTB-94, and subsequently assessed its effect on a rat model of monosodium iodoacetate (MIA)-induced OA.
Materials and Methods
Preparation of L. lancifolium extract
Dried Lilium lancifolium (LL) bulb was purchased from Humanherb Co., Ltd (Daegu, Korea) and pulverized to an appropriate size; 50% ethanol (5 to 10 times the weight of the bulbs) was added to the extraction vessel and stirred at 50℃. The obtained extract was concentrated in a rotary evaporator, and maltodextrin corresponding to 50% of the solid content was mixed and lyophilized to powder. The dried extract was dissolved in DMSO for cell experiments and in 0.5% carboxymethyl cellulose (CMC) aqueous solution for animal experiments. The voucher sample was kept in the Korean Yakult storage (voucher specimen 000-003).
Cell culture
HTB-94 (ATCC, VA, USA), a human chondrosarcoma cell line, was cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS) and 1% antibiotic-antimycotic solution (Gibco, MA, USA). The cells were maintained at 37℃ and 5% CO2 in a CO2 incubator until they reached 80-90% confluence, after which they were passaged. To confirm the anti-inflammatory effect of LL in articular cells, HTB-94 cells were seeded at 1.5 × 105 cells/well in 6-well plates, challenged with 10 ng/mL of IL-1β, and treated with or without LL for 24 h.
Animals
Specific pathogen-free (SPF) male Sprague-Dawley rats (8 weeks old) were purchased from a local vendor (RaonBio, Gyeonggi, Korea). Animals were housed under a controlled temperature of 23℃ and humidity of 50 ± 5% on a 12-h light/dark cycle (8 AM-8 PM). This study was approved by the Institutional Animal Care and Use Committee of Gyeonggi Bio Research Center (AEC-2016-00035-Y).
MIA-induced osteoarthritis
Osteoarthritis (OA) was induced with monosodium iodoacetate (MIA). Three ㎎ of MIA (Sigma-Aldrich, St. Louis, MO, USA) in a total volume of 50 μL (dissolved in 0.9% salin) was injected into the left knee of the animals, through the infrapatellar ligament, under anesthesia (Bove et al., 2003). The left knee of control side was injected with saline (total volume of 50 μL). The rats were randomly divided into 6 groups after 1 week of acclimatization (n = 6/group) (Table 1). Glucosamine hydrochloride (Sigma-Aldrich, St. Louis, MO, USA) was used as a positive control to compare the effects of LL; 0.5% CMC was used as a vehicle. All test substances were orally administered once a day for 5 weeks.
Table 1.
Test group composition and treatment
| Group (n = 8) | Treatment | Treatment Concentration | MIA |
| Control | Vehicle | - | X |
| MIA | Vehicle | - | O |
| MIA + Glu | Glucosamine | 200 mg/kg | O |
| MIA + LL | Lilium lancifolium | 200 mg/kg | O |
Hind paw weight-bearing distribution
Pain behavior was determined by the hind paw weight-bearing distribution, assessed using an incapacitance test meter (IITC Life Science, California, USA). The difference in strength of the left (ipsilateral) and right (contralateral) limbs was used as an index of discomfort in the knees. The results are presented as follows: % of weight on ipsilateral limb = (weight on ipsilateral limb/ weight on ipsilateral limb + weight on contralateral limb) × 100 (Bove et al., 2003). Rats were habituated for more than 15 min in a plastic box. A value of 50% of weight bearing indicated equal weight distribution on both limbs, and a value less than 50% represented an index of disease progression.
Blood analysis and measurement of cytokine levels
After the weight-bearing distribution test, the animals were sacrificed with CO2 gas, and whole blood was collected from the heart using a syringe. The blood was centrifuged at 4℃ for 15 min at 1000 × g to separate the serum, and the latter was stored at -80℃ until further analysis. IL-6, IL-8, LTB-4, MMP-9, PGE-2, and CRP concentrations were analyzed with an ELISA kit (Abcam, Cambridge, UK) according to the manufacturer’s instructions.
Histopathological examination
After blood collection, the knee of the animals was amputated, and the joint tissue was decalcified by placing it in a 10% formalin solution containing 10% EDTA. After decalcification, the specimens were embedded in paraffin and sectioned at a thickness of 7 μm. Slides were stained with hematoxylin and eosin, as well as Safranin-O, to observe the extent of cartilage-tissue destruction and inflammation.
Cartilage damage was assessed based on the depth and severity of injury. Depth was graded on a scale of 0-4, where 0 = normal; 1 = minimal, only affecting surface area; 2 = slight intrusion only in the middle upper area; 3 = medium intrusion into the middle area; 4 = significant intrusion into deep areas. The degree of tibial plateau involvement was scored as 1 (minimum), 2 (moderate), or 3 (severe). A modified Mankin system was used to score cartilage changes (Bar-Yehuda et al., 2009); the degree of inflammation was scored on a scale of 0-4, based on the degree of cellular infiltration into the tissue: 0, normal (no infiltration); 1, minimal inflammatory cell infiltration; 2, mild infiltration; 3, moderate infiltration; and 4, significant infiltration.
Statistical analysis
Statistical results were presented as mean ± standard error of the mean (SEM) with 95% confidence limits. Data were statistically compared using the one-tailed Student’s t-test, followed by unpaired comparison. All statistical analyses were performed using the GraphPad Prism 5 (San Diego, CA, USA) statistical software package; p values less than 0.05 were considered statistically significant.
Results
LL downregulated the level of pro-inflammatory cytokines in IL-1β-induced HTB-94 cells
We examined the effect of LL on the level of pro-inflammatory mediators in IL-1β-stimulated HTB-94 cells. HTB-94 cells were treated with 10 /mL and 100 /mL of LL, and 10 ng/mL of IL-1β for 24 h. HTB-94 cells treated with IL-1β had significantly increased levels of inflammatory mediators, such as IL-6, IL-8, and PGE-2. LL decreased the production of IL-6, IL-8, and PGE-2, induced by IL-1β, in a concentration-dependent manner; in particular, the production of IL-8 and PGE-2 was significantly decreased (Fig. 1A ~ C). High concentrations of IL-8 had been observed in the pathophysiological environment of OA (Chauffier et al., 2012), and PGE-2 is well known to stimulate matrix degradation in OA (Attur et al., 2008). Therefore, the observation of LL inhibiting the production of IL-8 and PGE-2 in the human chondrocyte cell line suggested the possibility of LL inhibiting the progression of OA.
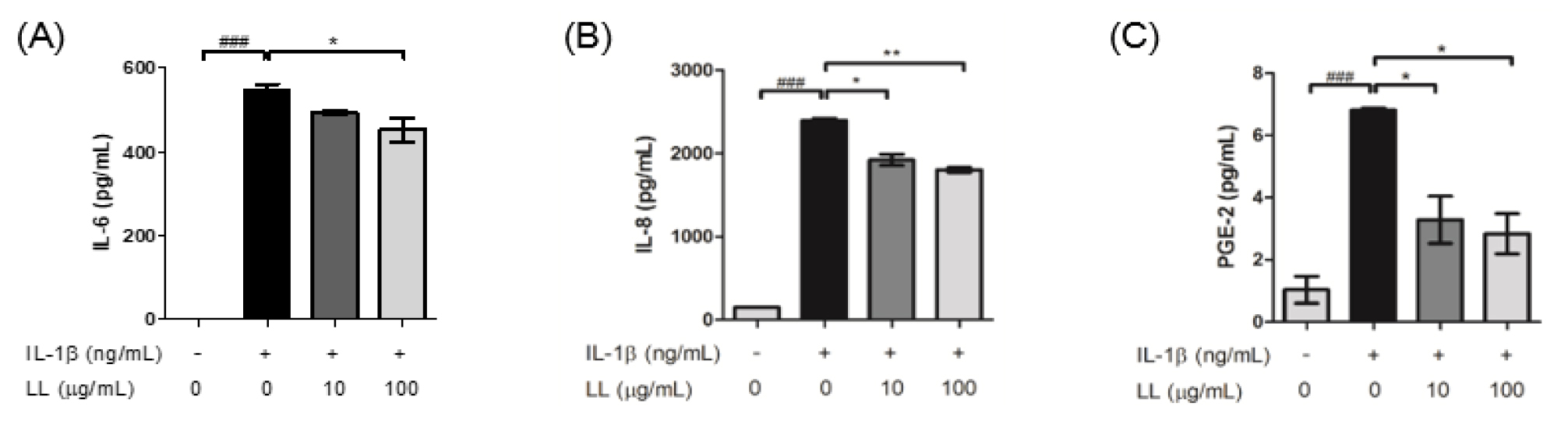
Fig. 1
Treatment with LL downregulated the level of pro-inflammatory cytokines in IL-1β-stimulated HTB-94 cells. (A) Treatment with LL changed IL-1β-induced IL-6 production. (B) Treatment with LL significantly reduced IL-8 production in IL-1β-stimulated HTB-94 cells in a dose-dependent manner. (C) LL extract reduced the PGE-2 production in IL-1β- stimulated HTB-94 cells in a dose-dependent manner. HTB-94 cells were challenged with IL-1β (10 ng/mL) and treated with or without LL (10 ㎍/mL and 100 ㎍/mL) for 24 h. Statistical analyses were processed by unpaired Student’s t-test. Data are represented as mean ± standard error of means (SEM). ###p < 0.001 compared to control; *p < 0.01 and **p < 0.05 compared to IL-1β-stimulated sample.
Oral administration of LL ameliorated the hind paw weight-bearing distribution in the rat model of MIA-induced OA
We next investigated the effects of LL on a rat model of MIA-induced OA. Weight bearing distribution was measured as a phenotype of OA progression and pain (Bove et al., 2003) on weeks 0, 3, and 5 (Fig. 2A). As shown in Fig. 2B, there was no significant difference across the groups in week 0. However, glucosamine hydrochloride (Glu)- and LL-treated groups showed significant increase in weight bearing capacity from week 3 (p < 0.05, p < 0.001). Joint discomfort lasted until week 5 in the MIA-induced group (p < 0.001). LL administration ameliorated the weight-bearing distribution in MIA-induced group (p < 0.05, p < 0.001) (Fig. 2D and E). Moreover, 5 weeks of LL administration had a better effect on weight-bearing distribution than that of Glu administration (p < 0.01).
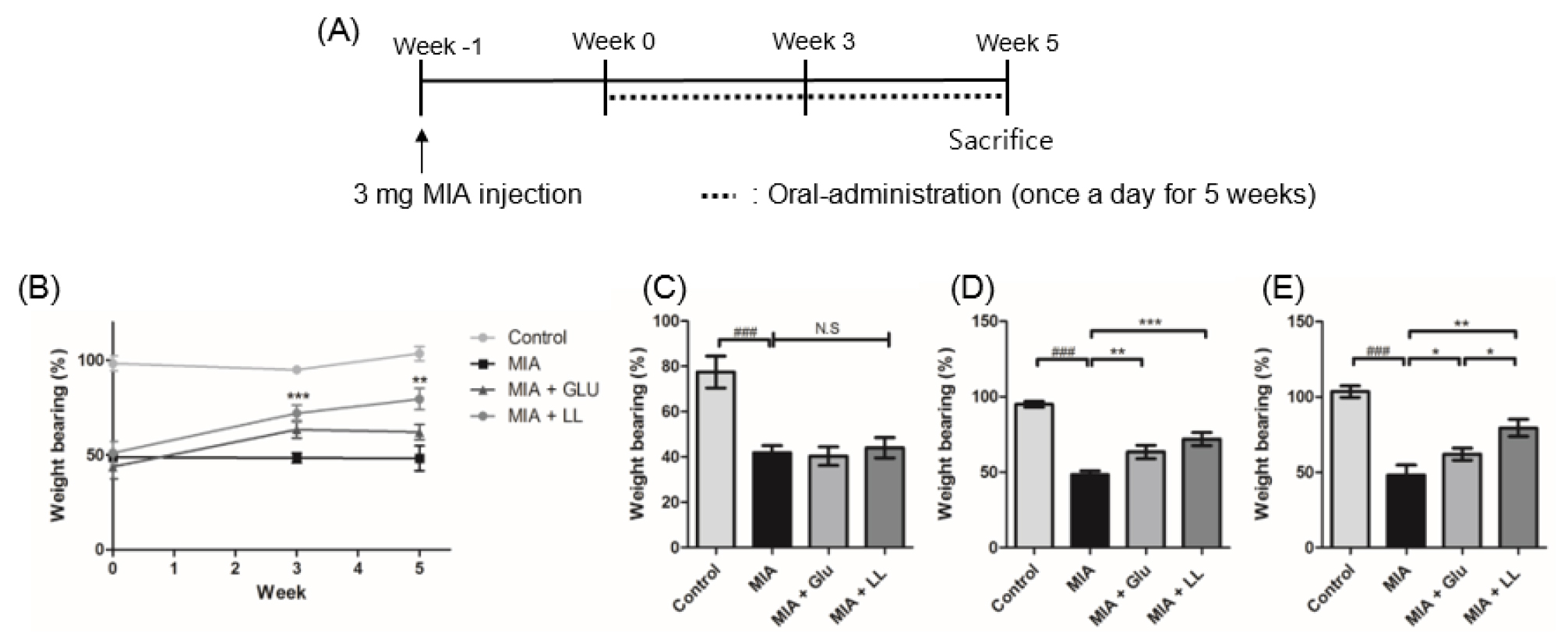
Fig. 2
Effect of LL on hind paw weight-bearing distribution in monosodium iodoacetate (MIA)-induced rats. (A) Scheme of MIA-induced osteoarthritis rat model. Oral administration of LL and Glu was conducted from week 0 to week 5. (B) The rats were assessed on an incapacitance meter on weeks 0, 3, and 5. Administration of LL significantly enhanced the weight-bearing distribution on weeks 3 and 6. (C) The percentage of weight-bearing on week 0. (D) The percentage of weight bearing on week 3. (E) The percentage of weight bearing on week 6. Statistical difference was processed by unpaired Student’s t-test. Data are represented as mean ± standard error of means (SEM) (n = 8/group) ###p < 0.001 compared to control group; *p < 0.01, **p < 0.05, and ***p < 0.001 compared to MIA group.
Administration of LL alleviated the level of inflammatory mediators in MIA-induced rats
Inflammatory cytokines play a destructive role in the cartilage and are eventually involved in the pathophysiological progress of OA (Wojdasiewicz et al., 2014). Therefore, we examined the effects of LL on the levels of pro-inflammatory cytokines and inflammatory mediators, such as TNF-α, IL-6, CRP, LTB-4, PGE-2, and MMP-9 in serum. The MIA group showed significantly increased levels of cytokines and mediators than the control group (p < 0.05, p < 0.001) (Fig. 3A ~ F). Increase in the levels of TNF-α and IL-6, which are known to induce acute immune response, was significantly prevented in the LL-administered MIA-induced group (p < 0.001) (Fig. 3A and B). The levels of systemic C-reactive protein (CRP) were significantly decreased in the LL-administered group (p < 0.05) (Fig. 3C). LL showed inhibitory effect on LTB-4 production (p < 0.05), whereas the MIA group showed a significant increase in LTB-4 values (p < 0.001) (Fig. 3D). PGE-2 release in serum was significantly inhibited in the LL-administered MIA group compared to that in the MIA group (p < 0.05) (Fig. 3E). Since MMPs are involved in joint destruction (Manicourt et al., 1994), we investigated the effect of LL on changes in concentration of MMPs in the serum. LL administration significantly alleviated the level of MMP-9 in MIA-induced rats (Fig. 3F), but did not affect the level of MMP-2 or MMP-1 (data not shown).
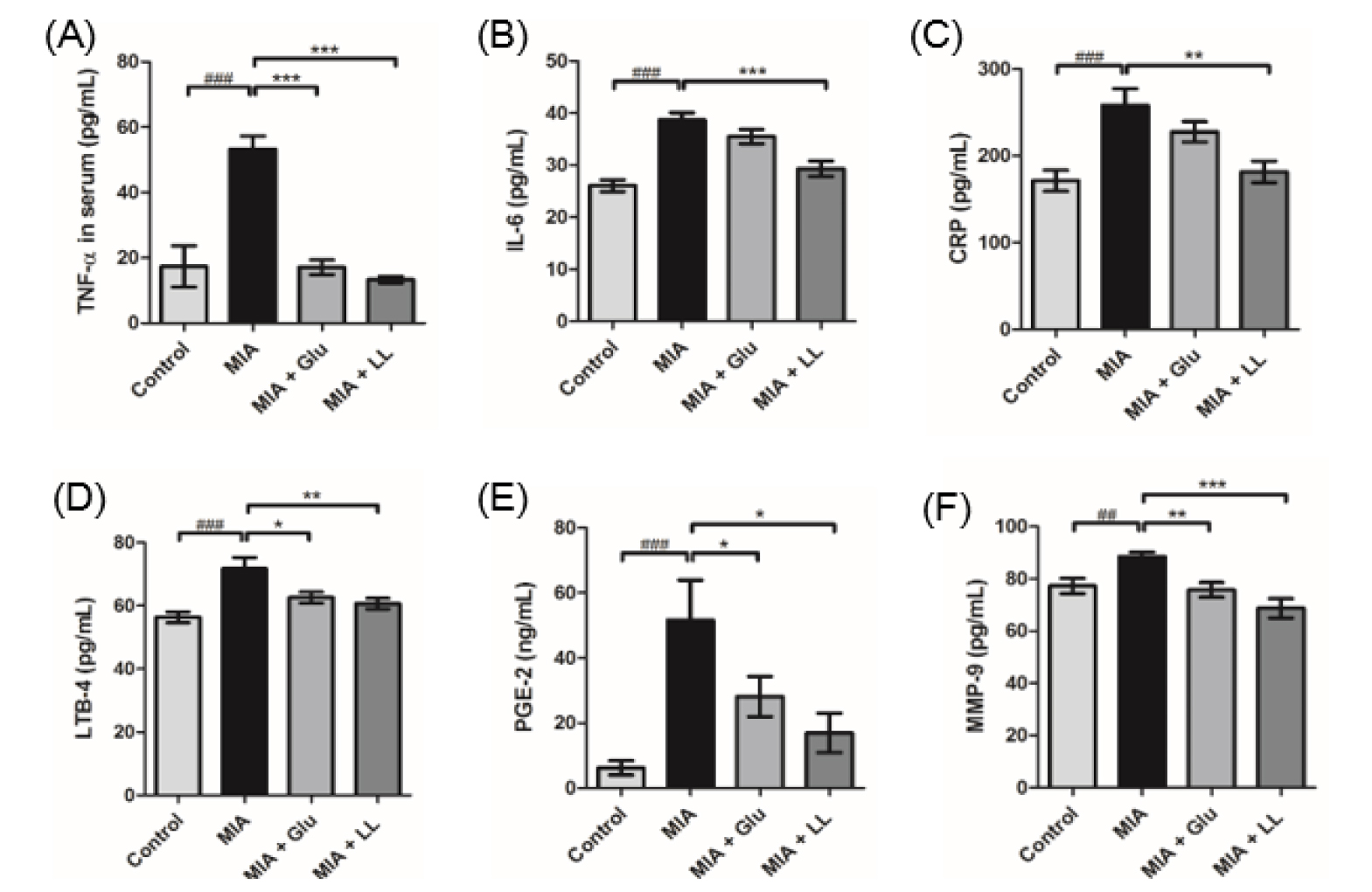
Fig. 3
Reduction of inflammatory cytokines and mediators by LL treatment. (A) LL significantly reduced the level of IL-6 in serum. (B) TNF-α level in serum was significantly decreased in LL-induced group. (C) LL inhibited the level of CRP in serum. (D) Oral administration of LL restored the level of LTB-4 similar to that in serum of the control group. (E) Chronic treatment of LL alleviated the amount of PGE-2 in serum. (F) The systemic level of MMP-9 was restored by chronic administration of LL. Statistical analyses were processed by unpaired Student’s t-test. Data are represented as mean ± standard error of means (SEM) (n = 8/group). ###p < 0.001 compared to the control group; *p < 0.01, **p < 0.05, and ***p < 0.001 compared to the MIA group.
LL alleviated the invasion of inflammatory cells in the joints of MIA-induced rats
H&E staining was performed to check the morphological changes in knee joint and inflammatory cell infiltration. Compared to the control group, the MIA-induced group showed remarkable bone destruction and inflammatory cell infiltration. On the other hand, in the LL-treated group, inflammatory cell infiltration was significantly reduced (Fig. 4A ~ D) and bone destruction tended to be suppressed (Fig. 4B ~ D). Damage of cartilage tissue was examined by staining the proteoglycan layer of cartilage with Safranin-O, and results showed the cartilage-tissue damage to be lesser in the LL-treated group than in the MIA group, although the difference was not significant (Fig. 4C and E).
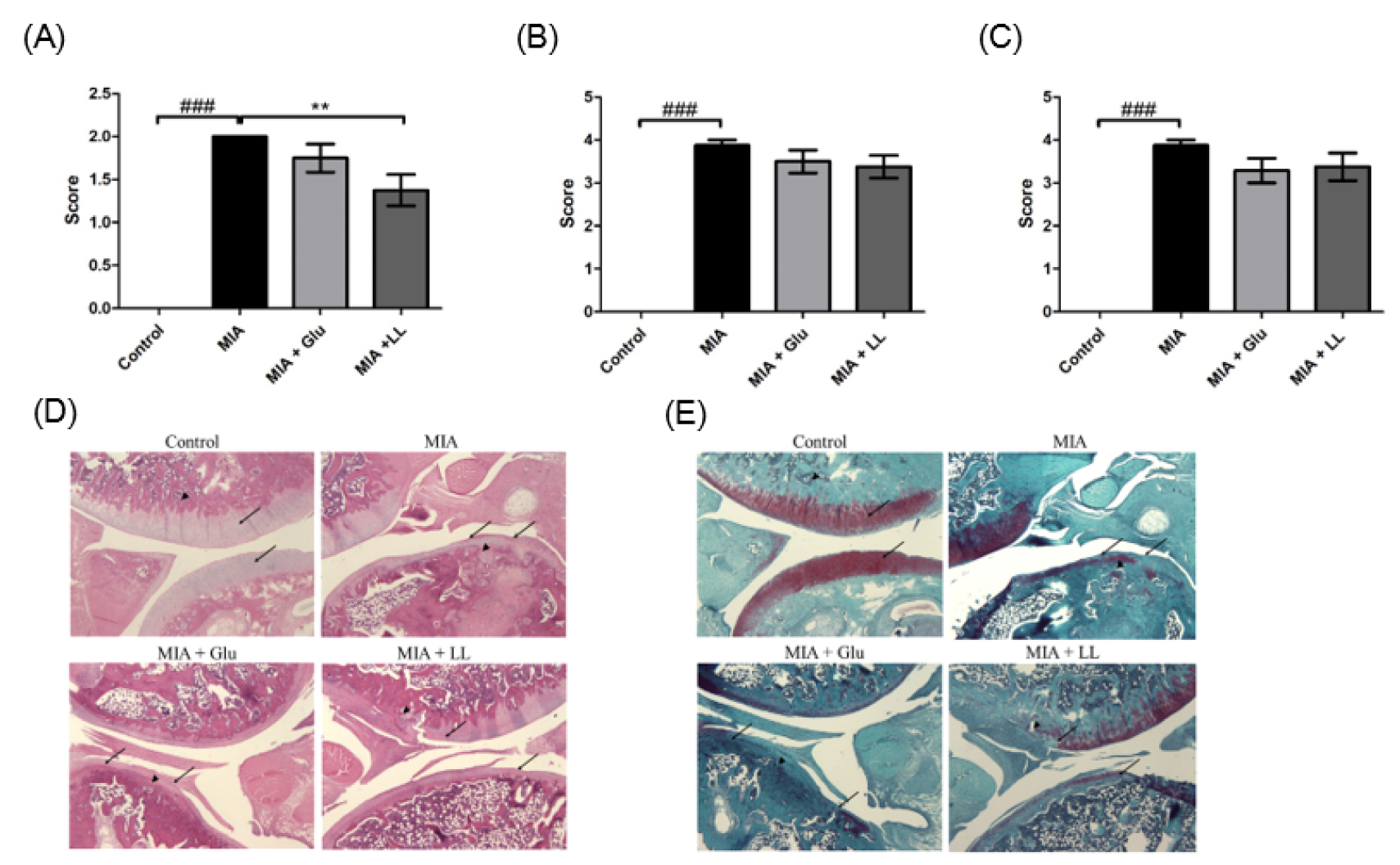
Fig. 4
Effect of LL on the pathology of joint tissues in rats with MIA-induced osteoarthritis. (A) Score of infiltrated inflammatory cells. (B) Score of bone destruction. (C) Score of cartilage degradation. (D) Representative image of H&E-stained joint tissue. (E) Representative image of Safranin-O-stained joint tissue.
Discussion
In recent years, with the development of medicine and nutrition, life expectancy has increased, and the elderly population above the age of 65 is increasing. As a result, many studies on senile diseases, such as diabetes, hypertension, Alzheimer's disease, and osteoarthritis are being actively conducted. Osteoarthritis is a representative disease, prevalence of which increases proportionally with age; however, it is generally regarded as a degenerative phenomenon due to aging, and therefore, overlooked with regard to treatment and disease management (Ashkavand et al., 2013). Currently, its treatment includes non-drug therapy, drug therapy, and surgical therapy; however, research is still ongoing to address problems such as, insufficient effectiveness or poor persistence, in clinical cases. Therefore, development of a new treatment for osteoarthritis that can prevent, as well as improve, osteoarthritis is imperative.
The aim of this study was to identify natural products that could relieve pain and inflammation in knee-joint damage. Our study, involving the treatment of IL-1β-stimulated human chondrosarcoma cells with LL, demonstrated effective inhibition of pro-inflammatory cytokine release. Therefore, chronic intake of LL was considered to be associated with the regulation of joint pain as well as systemic pro-inflammatory mediators.
Inflammatory mediators play crucial roles in tissue destruction in patients with OA (Lee et al., 2012). LL had been shown to exert anti-inflammatory (Kwon et al., 2010; Lee et al., 2013) and therapeutic effects in pulmonary inflammation and emphysema (Lee et al., 2013). IL-6 and IL-8 release in the joints of patients with OA can increase the release of cartilage-degradation mediators, such as MMPs (Grassel et al., 2010), and enhance bone loss in patients with OA (Kaneko et al., 2000). Our results suggested that LL down-regulates the inflammatory response, including IL-6, IL-8, and PGE-2, in IL-1β-stimulated HTB-94 cells. Moreover, LL reduced the levels of inflammatory cytokines and mediators in serum, and possibly inhibited the progression of MIA-induced OA in the knee joints.
Patients with OA are known to have disrupted collagen matrix in cartilage (Dejica et al., 2008; Stolz et al., 2009), besides an overall disruption of bone structure (Burr and Gallant, 2012), which can cause pain in the joints (Buckwalter and Brown, 2004). Moreover, patients with OA experience knee-joint pain and limited range of mobility (Buckwalter and Brown, 2004). LL administration effectively alleviated the weight-bearing distribution in animals with osteoarthritis. Therefore, LL seemed to have the potential to improve the loss of mobility caused by osteoarthritis. Both Glu and LL showed anti-inflammatory effects in animals with osteoarthritis; however, the latter had a greater impact on improving the weight-bearing distribution than the former.
These results collectively indicated that LL can modulate both pain and immune status. It can effectively ameliorate pain levels and systemic pro-inflammatory cytokine production. Supplementation with LL may be a potential therapeutic means in patients with OA; however, further studies on humans would be required in future.




