Introduction
Materials and Methods
Reagents and chemicals
Plant materials and extraction of lycopene
Extraction and analysis of lycopene
Statistical analysis
Results and Discussion
Fruit morphology analysis
Analysis of lycopene using spectrophotometric and HPLC methods Method validation
Lycopene levels in watermelon fruit flesh sample
Introduction
Watermelon is among the top economically important horticultural crops in South Korea (Park and Cho, 2012), with a production quantity of 534,401 tons in 2018 (Food and Agricultural Organization of the United Nations (FAO). Watermelon is a member of the Cucurbitaceae plant family (Wehner, 2008). It is a non-seasonal fruit mainly grown in areas with long frost-free warm periods. Watermelon fruits are produced in different sizes (icebox, small, medium, large, or giant), shapes (round, oval, blocky, or elongate), rind patterns (gray, narrow stripe, medium stripe, wide stripe, light solid, or dark solid), flesh colors (white, yellow, orange, pink, or red), and types (seeded or seedless) based on the cultivar. The red flesh, blocky shape, and large sized (8-11 ㎏) from seeded types, and red flesh, oval shape, and medium sized (5-8 ㎏) from seedless types are commercially popular cultivars (Wehner, 2008; Cho et al., 2014). Typical watermelon fruit types popular in Korea are of short oval type (8-9 ㎏/fruit), with high sugar content, green rind and dark stripe, and black rind without stripe (Park and Cho, 2012). Watermelon fruit approximately constitutes 68% flesh, 30% rind, and 2% seeds (Kumar et al., 2012), with the flesh mainly being water (about 93%) and small amounts of protein, fat, minerals, vitamins, carbohydrates, vitamin A, and lycopene (Perkins-Veazie et al., 2012; Wehner, 2008).
Lycopene is a lipophilic major carotenoid that gives a characteristic red color to fruits such as ripe tomato and pink grapefruit. Tomato is the main source of lycopene. Other sources include apricots, papaya, pink grapefruit, guava, and watermelon. Lycopene is a very efficient singlet oxygen quencher and potent scavenger of oxygen radicals among the group of carotenoids (Di Mascio et al., 1989; Islamian and Mehrali, 2015), and serves as an intermediate for the biosynthesis of other carotenoids (Sandmann, 1994). Lycopene provides important contributions to human health. Mechanistic and epidemiological studies shown that the consumption of dietary sources of lycopene has been associated with several chronic diseases such as cardiovascular diseases (CVD), obesity, type 2 diabetes, cancer, and neurodegenerative disorders (Saini et al., 2020; Stahl and Sies, 1997) and protection of harmful UV radiation (Stahl et al., 2006).
Consumption of watermelon fruits rich in lycopene content is proven to be beneficial for disease prevention as briefly described above. On the other hand, lycopene was reported to be a highly heritable trait with no cultivar x environment interaction (Wehner et al., 2017). Several morphological traits of Hungarian, Korean, and Turkish watermelon genetic resource have been examined previously (Huh et al., 2014; Solmaz and Sari, 2009; Szamosi et al., 2009). Studies on the lycopene content of watermelon germplasm collections are very scarce (Pinto et al., 2001) and mostly limited to commercial cultivars (Kalaivani, 2015; Nagal et al., 2012; Perkins-Veazie and Collins, 2003). Thus, investigating the lycopene profile, quality traits, and other morphological characters of watermelon genetic resources from various countries could provide important baseline data in breeding for increased lycopene levels thereby increasing the marketability of watermelon as a healthy food. The main objective of our work has been to determine the lycopene content of the flesh of watermelon fruit genetic resources by HPLC and spectrophotometric methods. We have also examined their fruit morphological diversity using some selected traits. One hundred and five watermelon fruit samples obtained from more than 22 countries and grown at the same agricultural conditions were used.
Materials and Methods
Reagents and chemicals
Lycopene standard (purity ≥ 90%), butylated hydroxytoluene (BHT), and tetrahydrofuran (THF) were purchased from Sigma-Aldrich (St. Louis, MO, USA). All other solvents and reagents used in extraction and analysis were of HPLC grade.
Plant materials and extraction of lycopene
Seeds of 105 watermelon genetic resources (100 germplasm collections and five commercial varieties) were obtained from the gene bank of the National Agrobiodiversity Center (NAC), Rural Development Administration (RDA), Jeonju, South Korea. Seeds were originated from 22+ countries. Seeds were sown at the research farm of the NAC, Gochang, Republic of Korea. RDA’s recommended cultural management practices for watermelon were followed in the experimental field. Fertilizers were applied (N-P2O5-K2O = 13.8-4.9-8.7 ㎏/10a) followed by RDA’s standard and drip irrigation tape was used for watering. Seeds were sowed on April 9, 2019, and grown in a nursery bed for 37 days. Seedlings (twelve plants from each accession) were transplanted at an area of 35㎝ × 300 ㎝ in a polyethylene film house equipped with insect net to prevent insect pollination. Plants of the same accession were grown in a single plot (plot area 12.6 ㎡). They were pollinated by hand and harvested after 45 days (on average) of pollination. Watermelon fruits were harvested at a fully mature stage, collected, stored in polyethylene bags, and immediately transferred to a -18℃ walk-in freezer until further processing. The flesh (mesocarp) of the watermelon fruit was carefully separated from the seeds and rind manually and the edible part was, juiced, frozen at -80℃, and lyophilized using vacuum freeze drier (Ilishibiobase, Rijssen, Netherlands). Lyophilized powdered samples were sealed to prevent moisture absorption and stored at -20℃ until analysis.
Extraction and analysis of lycopene
Lycopene was extracted from freeze-dried flesh of watermelon fruit based on modified protocol reported earlier by Fish et al. (2002). Briefly, 0.2 g of watermelon fruit flesh powder sample was transferred into a 50 mL conical tube; 3 mL distilled water was added into it; shaken until completely dissolved for five minutes. To this mixture, 20 mL solution containing 0.05% butylated hydroxytoluene (BHT) in acetone; 95% ethanol; and n-hexane in a ratio of 1:1:2 (V/V) was added; mixed for 15 minutes with a rotary shaker (180 rpm). The solution was held at room temperature for 5 minutes for the layers to separate. The upper (hexane) layer containing lycopene was used for HPLC and spectrophotometric analysis. All steps from watermelon sample preparation to extraction were performed in subdued lighting at room temperature.
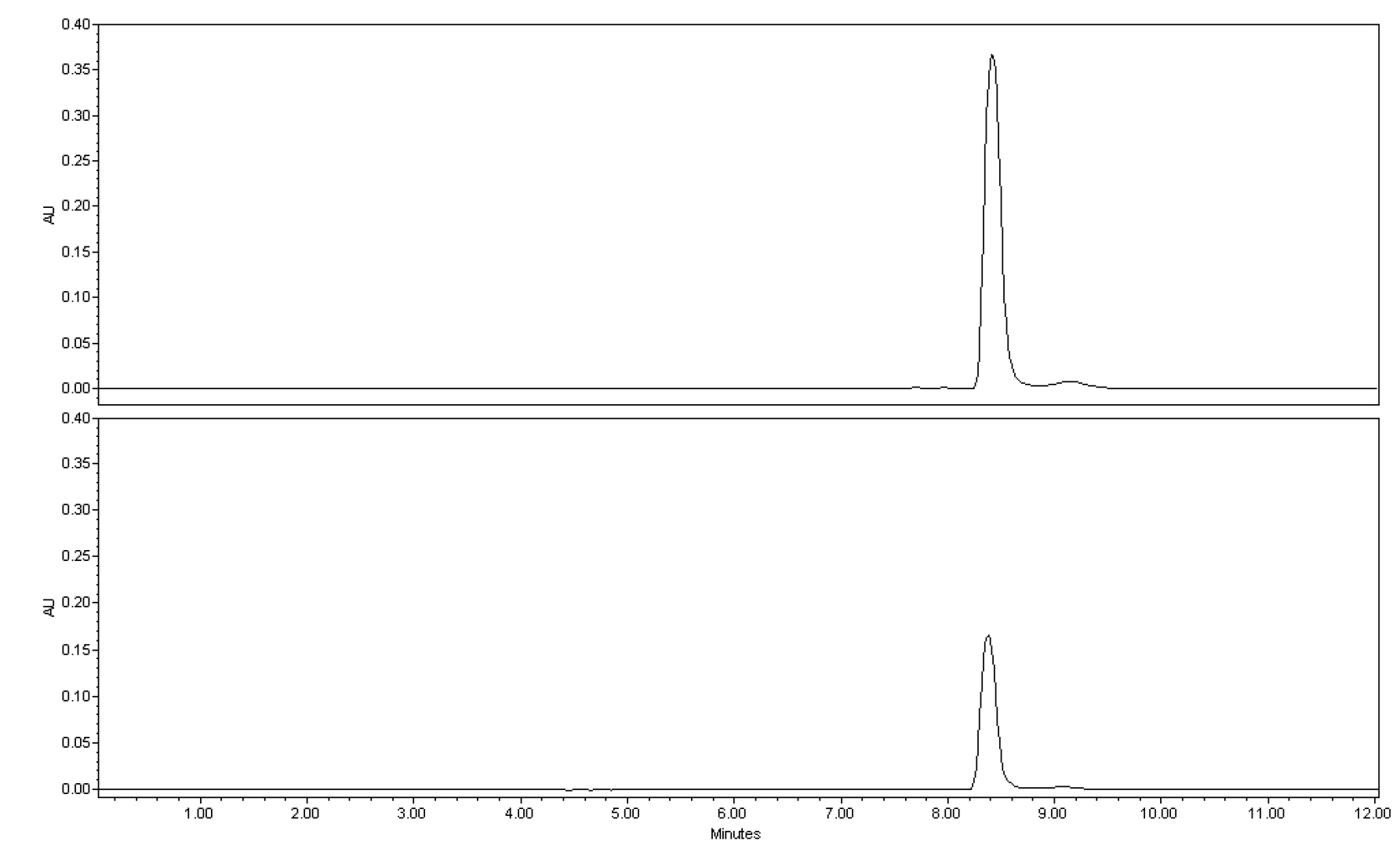
HPLC analysis of lycopene was done using Waters HPLC system equipped with a 2690 separation module and waters 996 diode array detector (Milford, MA, USA). The separation was conducted on Sunfire C18 column (4.6 × 250 ㎜, ID 5 ㎛) equipped with a C18 guard column (20 ㎜ × 4.6 ㎜, 5 ㎛) obtained from Waters (Milford, MA, USA). The elution mode was isocratic 100% acetone at a flow rate of 0.5 mL/min, injection volume 10 µL, analysis time 12 min. The detection wavelength was set at 503 ㎚. Results were expressed in microgram/gram (㎍/g) on a dry weight basis and calculated using an external calibration curve equation (Y = 176483X - 110142 R2 = 0.9994; Y = peak area, X = lycopene standard concentration) prepared using serial dilutions of lycopene standard. Lycopene was eluted at a retention time (tR) of 8.4 minutes (Fig. 1).
Spectrophotometric determination of lycopene was done using a Shimadzu UV-1601 UV-Visible spectrophotometer (Tokyo, Japan) and VERSAmax tunable microplate reader spectrophotometer (Molecular Devices, California, USA). The absorbance of the extract (hexane layer) (1000 µL and 200 µL in Shimadzu UV-1601 UV-Visible (UV) and VERSAmax tunable microplate reader (MR) spectrophotometers, respectively) was measured against hexane blank at 503 ㎚ and results were calculated based on an external calibration curve constructed from the absorbance of serial dilutions of lycopene concentration. The calibration curves were Y = 0.226X + 0.0443 (R2 = 0.9989) and Y = 0.0653X + 0.0658 (R2= 0.9995), where Y is absorbance and X is concentration of lycopene standard, for UV/Vis spectrophotometer (UV) and microplate reader (MR) spectrophotometer, respectively. Final results were expressed as ㎍/g on a dry weight basis.
Statistical analysis
Experiments were conducted in biological triplicates. Lycopene contents are reported as mean ± standard deviation. Quantitative morphological characters were reported as averages of values from 10 to 12 watermelon fruits. Correlation analysis was conducted using SPSS V25 statistical program (SPSS Inc., Chicago, The USA). PCA analysis was conducted using R-program (Version 3.6.1, RStudio, Inc.,).
Results and Discussion
Fruit morphology analysis
Based on the International Union for the Protection of New Varieties of Plants (UPOV) for watermelon fruits (International Union for the Protection of New Varieties of Plants (UPOV), 2019), 14 phenotypic characters of watermelon fruit were assessed. The size of pistil scar (SPS), the width of stripes (WS), weight of fruit (WF), length of fruit (LF), width of fruit (WIF), the thickness of pericarp (TP), and soluble solids content (SSC) were measured with the help of a meter, digital caliper, digital balance, and a handheld electronic refractometer as required. Other qualitative characters were recorded on the field and inside the laboratory. Quantitative characters of 105 watermelon fruit samples are presented in Table 1. Other qualitative characters including fruit shape in longitudinal section, ground color of skin, the intensity of the green color of skin, fruit shape at the apical part, grooving distribution, conspicuousness of stripes, and main color of the flesh are presented in a supplementary file (Appendix 1).
Table 1.
Lycopene content and some selected quantitative morphological characters in watermelon fruit flesh samples
Watermelon fruits have shown a wide range of diversity in fruit size, shape, rind color, flesh color, rind patterns, weight, and SSC. Most samples (94.3%) had green ground skin color and the remaining were white-colored. The intensity of the green color of the skin showed a wide variety which was described from very light to very dark. Most of the resources were shaped circular (60.6%) and broad elliptic (28.8%) in the longitudinal section. Truncate and little rounded shaped fruits at the apical part comprised 90.5% of the resources. Fruit grooves are absent in 85% of the fruits and stripes were conspicuous in 66.7% of the resources. The main color of the flesh was mostly pink, red, and a mixture of the two (96 of 105). The remaining were either yellow or orange. In agreement with this study, yellow and white flesh color were quite scarce in previous reports (Sari et al., 2007; Solmaz and Sari, 2009; Szamosi eet al., 2009).
The width of the stripes (0.0 to 29.8 ㎜), size of the pistil scar (2.0 to 32.6 ㎜), and thickness of the pericarp (4.6 to 22.6 ㎜) showed wide diversity among the entire population. The fruit weight, soluble solids content, length, and width of watermelon fruits were also showed quite a range of diversity but the medium character was common in most of the accessions. The average values of soluble solids, rind thickness, and length and width of fruits are comparable with previous reports (Hartman et al., 2019; Huh et al., 2014; Solmaz and Sari, 2009; Soteriou et al., 2014; Szamosi et al., 2009; Wehner et al., 2017), however, the fruit weight and size of pistil scar of the resources investigated in this study are quite higher. Some of the characters had a significant correlation with each other. The weight of the fruit was significantly positively correlated with length and width, which is quite expected. Other important significant correlations (p < 0.01) observed are between the size of pistil scar and weight, length, and width of fruit, where the size of the pistil scar was negatively correlated with the former two characters and positively correlated with the later. Watermelon fruits that had a higher thickness of rind were found to exhibit less SSC (Table 2).
Table 2.
Pearson’s correlation coefficient of traits for watermelon fruits samples
The origins of the genetic resources showed to affect the morphological characteristics of some resources to a certain extent. For instance, KOR origin watermelon fruits were characterized by less size of pistil scar, conspicuous longer width of stripes, intermediate SSC, red/pink colored flesh, absence of grooves, little rounded shape at the apical, and varied shape at the longitudinal section. On the other hand, in watermelon fruits originated from IRQ, KGZ, MNG, and URY stripes are absent, while fruits from ITA and BRA exhibited longer width of stripes. The USA, RUS, TJK, TKM, TWN, and URY originated fruits had generally higher SSC compared to other origins. IND, BRA, USA, and CHN origin fruits were larger in weight and length.
Analysis of lycopene using spectrophotometric and HPLC methods Method validation
The extraction of lycopene was conducted using a mixture of solvents and reagents according the previously reported method (Fish et al., 2002), with some modification. In our experiment, distilled water was added at the first stage of the extraction step as opposed to the procedure followed by Fish et al. (2002). Watermelon fruit flesh sample contains a high level of sugar which is less soluble in organic solvents and form aggregates that make dissolution slow. Preliminary experiments showed that the lycopene content highly varied with the degree of dissolution of the sample with an organic solvent added at the first step. The addition of water at the primary stage allowed a better dissolution of the extraction mixture.
Calibration curves were prepared from an average of three independent lycopene standard solutions of serial dilutions. According to the calibration experiment, the UV, MR, and HPLC methods showed linearity in the range between 0.195 and 6.125, 0.781 and 50.00, and 0.195 and 50 ㎍/mL of lycopene concentrations, respectively. Recovery test, inter- and intra-day precision, limit of detection (LOD) and limit of quantification (LOQ) of lycopene analysis were performed using two watermelon fruit samples. The recovery test verified the efficiency of the methods for extraction and analysis of citrulline. The data obtained by spiking 50 to 300 ㎎ standard of lycopene showed a mean recovery of 101.85 to 109.67%, 94.26 to 100.07%, and 93.84 to 107.93% using UV, MR, and HPLC methods, respectively, suggesting the reliability and accuracy of the methods. Recovery test results are tabulated in Table 3.
Table 3.
Recovery test results of HPLC and spectrophotometric methods using two watermelon fruit samples
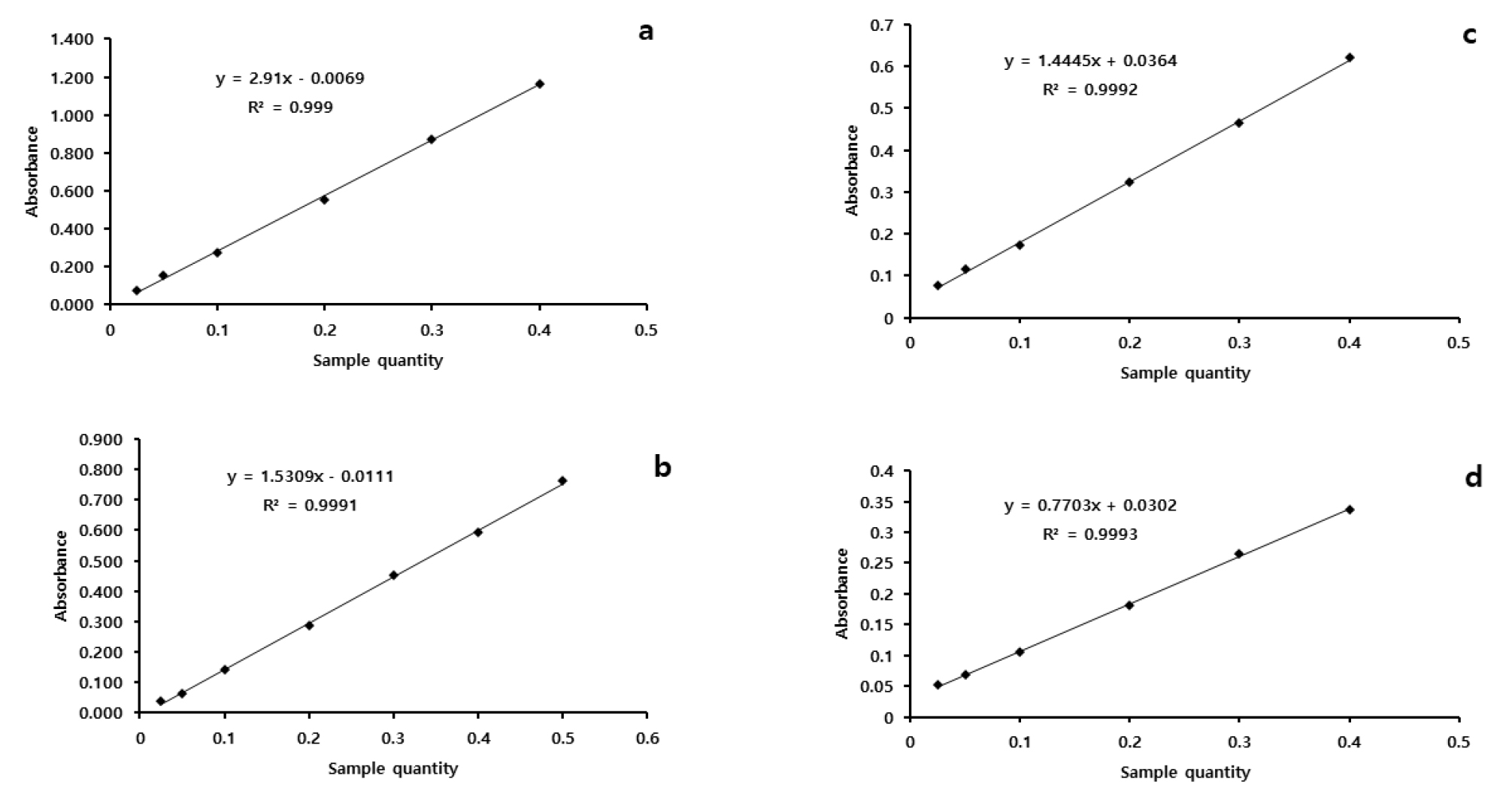
The precision of the method was determined as the percentage of the ratio of the standard deviation to the mean value (relative standard deviation, RSD) of inter-day (n=5) and intra-day (n = 5) analysis. The inter-and intra-day precision results are summarized in Table 4. The limit of detection (LOD, 3.3*σ/S; σ indicates the standard deviation of Y- intercept while S stands for the slope of the calibration curve) and the limit of quantification (LOQ, 10*σ/S) were 0.0063, 0.0082, 0.4258 ㎍/mL and 0.0189, 0.0247, and 1.2775 ㎍/mL, for UV, MR, and HPLC methods, respectively, demonstrating each of the methods were efficiently sensitive enough to detect the amount of lycopene in watermelon samples. The effect of sample size (sample to solvent ratio) on the response of the instruments was evaluated in two watermelon fruit samples for spectrophotometric methods (Fig. 2). A linear response was exhibited in the range of 0.2 to 0.4 g dry weight of watermelon fruit samples in both instruments.
Table 4.
Inter- and intra-day precision results of HPLC and spectrophotometric methods using two representative watermelon fruit samples
Lycopene levels in watermelon fruit flesh sample
Lycopene content varied widely among genetic resources and across red, pink/red, pink, orange, and yellow-fleshed watermelon fruits with red and pink/red-fleshed genetic resources exhibiting relatively higher lycopene content (Table 1). The lycopene content varied between 41.37 and 182.82 ㎍/g, 2.81 and 163.72 ㎍/g, and 3.54 and 255.47 ㎍/g using HPLC, UV and MR methods, respectively. Of the genetic resources evaluated in this study, the red-fleshed accessions IT 204167 (S/No 45) and IT 174803 (S/No 8) had the highest levels of lycopene content compared to other accessions. In five yellow-fleshed accessions (S/No 55, 67, 84, and 94), lycopene was below the detectable amount using all the three methods employed. When averaged for all colors, the content of lycopene decreased in the order of red, pink/red, pink, orange, and yellow (Table 5). A wide range of variability in lycopene content among cultigens was also reported previously (Nagal et al., 2012; Perkins-Veazie et al., 2001; Yoo et al., 2012). In a recent study of seven commercial cultivars and one breeding line watermelon cultigens, scarlet red-fleshed watermelon exhibited the highest lycopene, followed by deep red and coral red-fleshed cultigens (Wehner et al., 2017).
Table 5.
The average contents of lycopene in red, pink/red, pink, orange, and yellow fleshed watermelon samples
| Flesh color | Number of samples | Average lycopene content (㎍/g) | ||
| HPLC | UV | MR | ||
| Yellow | 4 | 0.0 | 0.0 | 0.0 |
| Orange | 5 | 63.4 | 30.1 | 44.8 |
| Pink | 30 | 73.3 | 40.2 | 63.1 |
| Pink/red | 37 | 88.0 | 59.8 | 91.0 |
| Red | 29 | 98.3 | 72.0 | 110.2 |
Lycopene content had a significant positive correlation with soluble solid content. However, no correlations were detected between lycopene and other quantitative fruit morphological characters (Table 2). In concordance with this study, Perkins-Veazie et al. (2001) and Davis et al. (2013) reported a positive correlation between lycopene and soluble solids while other morphological traits such as fruit weight, length, and width were uncorrelated with lycopene content. In contrast, Wehner et al. (2017) recorded a negative correlation between lycopene and soluble solids, suggesting the canary yellow cultigens containing higher soluble solids and lower lycopene could play a significant role to the correlation coefficient. Those authors have studied the correlation between lycopene and soluble solid content using a small number of samples (less than eight). Our study, which was conducted using a large number of samples, could confirm the lycopene and soluble solid content are correlated with each other.
Various analytical methods have been employed for analysis of lycopene content in watermelon and other lycopene containing sources (Son et al., 2011). Liquid chromatographic and spectrophotometric methods at 503 ㎚, which of the carotenoids known in watermelon only lycopene appears to absorb at this wavelength, have been widely employed by various authors (Davis et al., 2003; Fish eet al., 2002; Liu et al., 2010; Nagal et al., 2012; Perkins-Veazie et al., 2001; Suica-Bunghez et al., 2011). Lycopene is a tetraterpene structure consisting of eight isoprene units forming a conjugated double bond system that are solely composed of carbon and hydrogen. The double bond system (chromophore) of the lycopene allows the absorption of light in the range between 420 and 520 ㎚ (Tan and Soderstrom, 1989). In this study, we have used two types of instruments, Shimadzu UV-1601 UV-visible (UV) and VERSAmax tunable microplate reader (MR) spectrophotometers, to determine lycopene spectrophotometrically. The latter provided a linear response at higher concentrations (between 0.781 and 50.00 ㎍/mL) while the former had a working range at low concentrations of lycopene (0.195 and 6.125 ㎍/mL). The lycopene content measured using the UV-visible spectrophotometer were lower compared to the microplate reader and HPLC. Fourier Transform Infrared (FTIR) spectroscopy has also been described as a simple analytical method for rapid, accurate, sensitive, and reliable determination of lycopene in tomatoes and other food products with minimal sample preparation (Shahzad et al., 2014; Suica-Bunghez et al., 2011). FTIR enables the identification of the functional groups in lycopene showing typical bands arising as a result of C-H stretching (3000- 2800 ㎝-1), C-H bending (1477-1400 ㎝-1), and C-C and C-C-H stretching (1400-1100 ㎝-1). Another advanced analytical method, GC-MS, used for the identification of different volatile chemical constituents including lycopene in watermelon (Kalaivani, 2015). Lycopene concentration has been also been estimated in watermelon puree from their visible reflectance spectra acquired using fiber optic spectrometer in the wavelength range of 500-750 ㎚ (Choudhary et al., 2009).
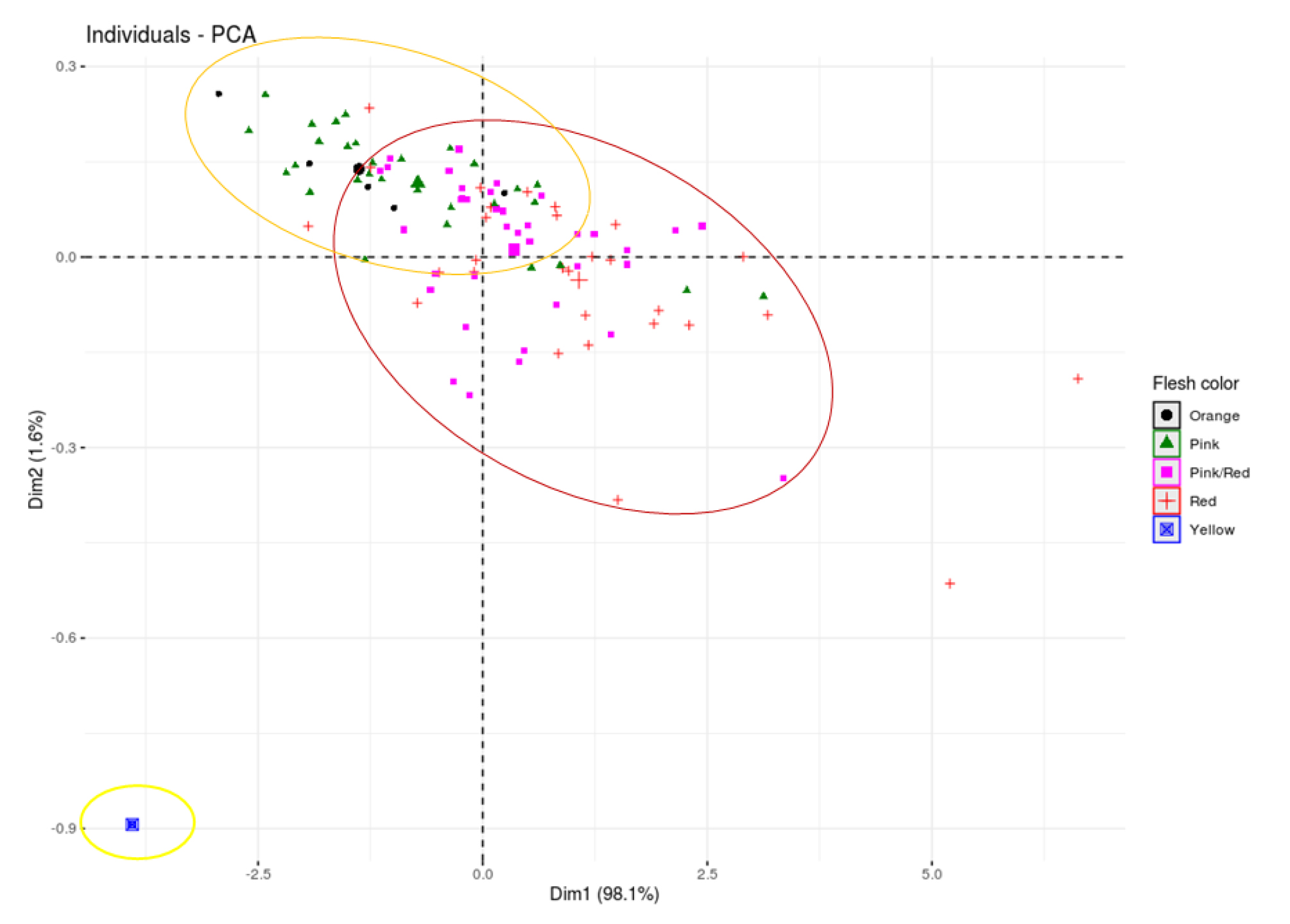
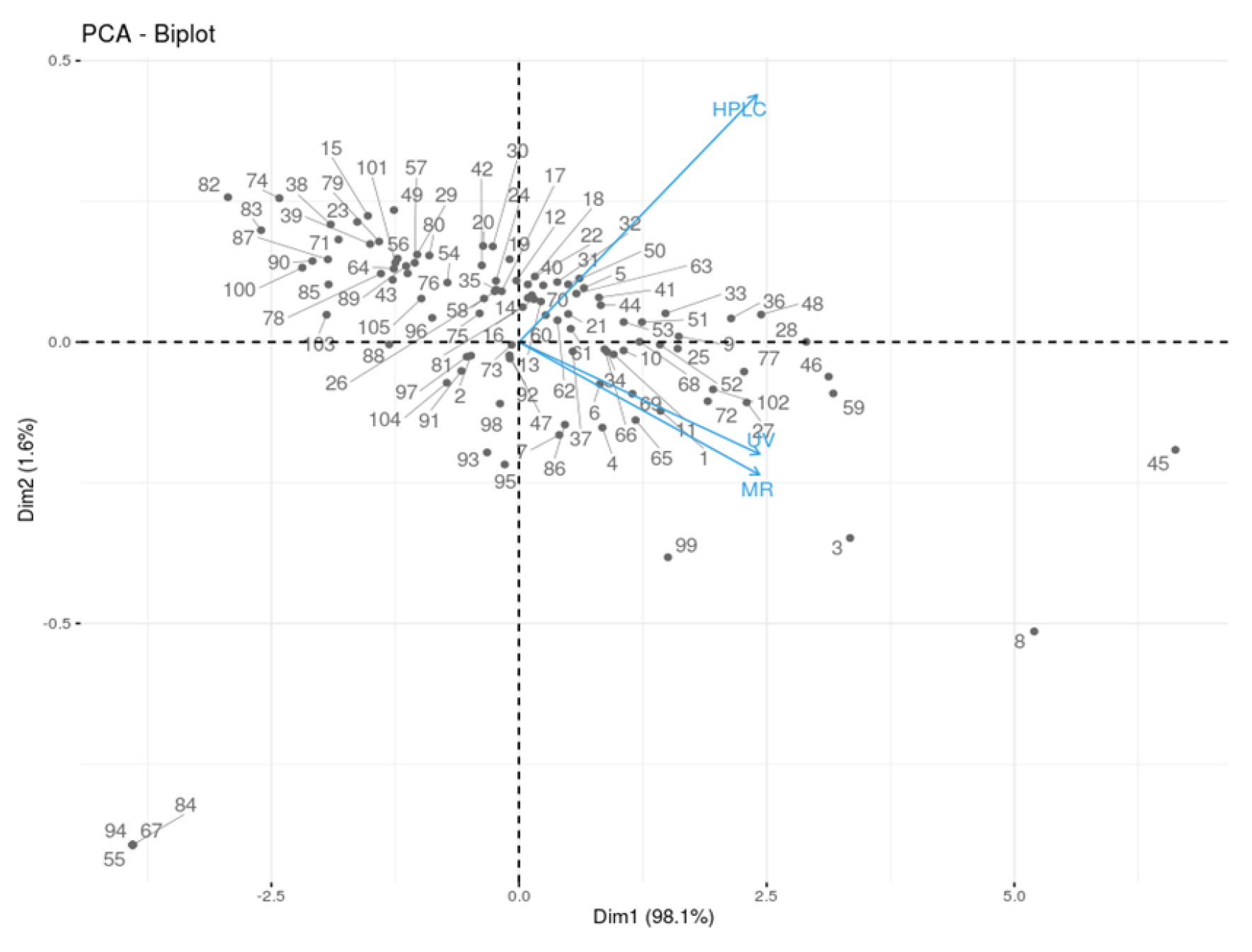
In an attempt to group the watermelon fruit samples based on their flesh color, Principal Component Analysis (PCA) was conducted (Figs 3 and 4). The first two principal components (Dim1 and Dim2) contributed 98.1% and 1.6% of the variations. As can be seen in the PCA plots, the individual fruit samples were seen distributed throughout the four quadrants with no significant groupings except in some cases. For example, four yellow-fleshed watermelon fruits (S/No 55, 67, 84, 94), seen at the lower negative quadrant of Dim1, were separated from the entire separation due to the absence of lycopene in a detectable amount. Other notable resources such as S/No 45, 8, 3, and 99 are described by their significantly high amount of lycopene. All were red-fleshed except S/No 3 which was described to be a mixture of pink and red. The PCA-Biplot and the individual PCA plot generated based on flesh color showed that most of the red- and red/pink-fleshed samples were co-located with lycopene content at the right side, suggesting the red- and red/pink-fleshed fruit samples contained a higher amount of lycopene compared to pink and orange fleshed samples.
In conclusion, our study demonstrated high diversity exists in fruit morphological traits and lycopene content of the germplasm collections. More than 90% of the genetic resources were red and pink fleshed. Fruits with higher thickness of rind were found to exhibit less soluble solid content (SSC). Korean origin fruits were characterized by intermediate SSC while The USA, RUS, TJK, TKM, TWN, and URY originated fruits had generally higher SSC. The red- and pink-fleshed fruits had the highest levels of lycopene content compared to the yellow and orange fleshed resources. Lycopene content had a significant positive correlation with SSC, however, no correlations were detected between lycopene and other quantitative fruit morphological characters. Owing to the increasing human population, there are increasing demands of high yielding, diseases resistant, and highly nutritive food crops including watermelon. For this reason, conservation and collection of genetically diverse watermelon germplasm are essential activities for the success of watermelon research and cultivation programs. As the modern desert watermelon cultivars share a narrow genetic base in terms of important quality traits, morphological characters, and phytonutrients; identifying and incorporating new resources with diverse characters into the cultivated crop is crucial. Utilizing the plethora of resources available within the watermelon germplasm collections in gene banks provides a means to enable the maintenance and improvement of the current levels of production, marketability, and health-related benefit of watermelon fruits. Our study results are important to plan future germplasm sampling and evaluation studies and also provide baseline data that could be beneficial for a future breeding program. In addition to quality trait and phytonutrient analysis, molecular marker characterization and identification of sources of resistance to diseases and pests are also important for genetic diversity analysis of watermelon germplasm.