Introduction
Materials and Methods
Strains and culture conditions
Plant extracts and chemicals (toxicants)
Mass screening of plant extracts
Fractionation of plant extract
Field test of control rate of rice blast disease
Statistical analysis
Results and Discussion
Mycelial growth inhibitory effect of extract of A. asphodeloides
Fractions of A. asphodeloides extract have antifungal activities
Control effect for rice blast disease of the extract of A. asphodeloides
Introduction
Rice blast disease occurs by filamentous pathogenic fungus of ascomycete, Magnaporthe oryzae. Though, rice is the most important crop plant, but the decrease of the rice crop production causes serious problems of food supply and starvations in worldwide regions. The disease is divided into seedling blast, leaf blast, and neck blast that cause the biggest damages to rice itself and crop production (Dean et al., 2005; Ribot et al., 2008). It occurs in almost all areas of the world including Korea where the rice is domestically cultivated as a crop. Many kinds of chemical pesticides are being used for the control of the disease, but their overuses cause the drastic environmental problems by their persistent toxicity. Consequently, the overuses of chemical pesticide cause expenditures for the disease control, and importantly, the drastic decrease of rice crop production, and environmental pollutions (Couch et al., 2005). In present, screening of pesticide resistant varieties of the M. oryzae and the improvement of fertilization method are being adopted for the control of rice blast disease, but chemical control by pesticides are being preferred domestically in Korea because of their high control efficiencies for their respective environment (Skamnioti and Gurr, 2009). But it has been gradually known that the efficiency of chemical controls was greatly threatened, and its usages are being restricted by the increase of the harms of environmental toxicity, and the advent of resistant fungal races against various pesticides were caused by pathogenically mutated new races (Chen et al., 2005; Koh et al., 1986). Those results have been caused by the fact that microorganisms including M. oryzae, generally have basic capabilities to resist to various natural toxicants, (Dixon et al., 1994; Dixon and Lamb, 1990; Cho et al., 2018, Jang et al., 2018) against chemical pesticides are also observed in various pathogenic fungi of rice plant to survive the toxic ecological environments. As for the molecular mechanisms of the pesticide resistances, a mutation or overproduction of target protein, and detoxification of treated pesticides are reported so far (Gulshan and Moye-Rowley, 2007). Recently, ABC (ATP Binding Cassette) transporter family proteins of plant pathogenic fungi are reported to be involved in multidrug resistance (MDR) mechanism by which chemically unrelated pesticides or compounds secreted from plant cell out of their cells are effluxed for the defense of themselves (Davidson et al., 2008; Di Pietro et al., 1999; Li and Wang, 2006; Zolnerciks et al., 2011). In the case of M. oryzae, about forty kinds of ABC transporters genes are known to be existed in its genome which was firstly revealed complete nucleotide sequences of genome in plant pathogens and are estimated to exist 11,109 genes (Dean et al., 2001; Jones and George, 2004), so the M. oryzae is being recognized as the model of molecular researches of plant pathogens. In our previous reports, ABC transporter, ABC2, of M. oryzae are involved in pesticide resistance by the pesticide efflux mechanism (Lee et al., 2005), and also we recently proved that the plant extract of Lysimachai foenum gracum Herba inhibit the mycelial growth of M. oryzae, and it caused decrease of ABC transporter genes expression of M. oryzae. Furthermore, when the extract is combinationally treated to mycelia of M. oryzae with blasticidin S, a pesticide for rice blast disease, IC50 of blasticidin S was dramatically decreased and the decrease of ABC transporter gene expression was also simultaneously accompanied (Lee, 2016). From those results, we found that the plant extract can increase the sensitivity of M. oryzae for blasticidin S through the inhibition of ABC transporter expressions of M. oryzae. There are also early supporting reports that antifungal activity of L. foenum gracum Herba inhibits the mycelial growth of M. oryzae and Botrytis cinerea (Iida et al., 1999; Park et al., 2003). Based on our previous reports and our preliminary experimental result for the extracts of L. foenum gracum Herba, we concluded that unknown active ingredients of L. foenum gracum Herba could contributed to the inhibitory mycelial growth of M. oryzae. Likewise, it is noticeable to the enormous variety of plant extracts that might have abilities of overcoming pesticide resistance of plant pathogens like M. oryzae.
In this study, we focused on the enormous diversity of plant extract from various species, so additionally screened 700 kinds of plant extracts on PDA (Potato Dextrose Agar) medium with high dose (500 ㎍/㎖) of plant extracts from various species. To determine the mycelial growth inhibitory effects of each plant extract on M. oryzae, we measured their mycelial radii after 5 day incubations after their treatments. Among them, the extract of A. asphodeloides showed the highest inhibitory effect. Fractionation experiment was also performed for the extract to determine organic solvent fraction containing putative active ingredients concerned with mycelial growth inhibitory effect. Finally, we certified the control rate for the rice blast disease in rice crop field test to verify the field adaptability of A. asphodeloides extract that could be a new candidate of biopesticide for the control of rice blast disease. In this study, we are sure that our experimental data can provide the possibility of plant extract of the diverse species as being a new material for the development of a new biopesticides.
Materials and Methods
Strains and culture conditions
M. oryzae strain KJ201 was distributed by KNRRC (Korea National Research Resource Center, Seoul national university) and was cultured on potato dextrose agar (PDA, Potato dextrose broth (Acumedia) 24 g, agar 1.5 g, per liter) and incubated at 28℃ incubator (Wooshin. 9109). All the pesticides and plant extracts were treated 3 hrs earlier in PDA.
Plant extracts and chemicals (toxicants)
About 700 kinds of plant extracts were supplied from Plant Extract Bank in Korea by dry weight of 20 ㎎ (Table 1). The total extract and solvent fractions was prepared from the leaves of A. asphodeloides.
Mass screening of plant extracts
1) Test of mycelial growth inhibition
The mycelial growth inhibition for M. oryzae was tested on PDA media in 24 well plates (SPL Life Science) containing each of plant extracts. To determine the mycelial growth inhibitory effect of 700 kinds of plant extracts, Mycelia of M. oryzae were roundly cut by Cork borer (KOREA Ace, 5 ㎜) and were placed on center of PDA media amended by 500 ㎍/㎖ of plant extracts and incubated for five days at 28℃ incubator (DAEHAN LABTECH. LSI-3016R).
2) IC50 determination of total extract
Plant extracts showing below 0.3 ㎝ of mycelial radii on PDA media were selected for further determination of IC50 (50% inhibition concentration). IC50 was calculated by methods of Valgas et al. (2007) and Magaldia et al. (2004) and the value of mycelial radii measured on PDA media amended by A. asphodeloides extracts or other pesticides were used for the IC50 determination by five day incubations at 28℃ incubator. All tests were performed in triplicate.
Fractionation of plant extract
1) Isolation of total extract of A. asphodeloides
1,000 g of completely dried leaves of A. asphodeloides was grinded by grinder (HMF-340, Hanil, Korea) and was mixed with absolute methanol (HPLC grade, Burdick & Jackson) with changing the solvent three times through the incubation at 50℃ for 24 hrs to extract the plant constituents completely. Methanol extract was filtrated by Whatmman, No. 2 filter and was vaporized by Rotary evaporator N-1000 (Eyela, Japan) at 45℃ for a week.
2) Preparation of fraction
Methanol concentrates of total extract of A. asphodeloides were suspended by 200 ㎖ of sterilized water, and was orderly fractionated by adding equal volume of hexane, chloroform, ethyl acetate and n-butanol and each of them was concentrated by vaporization.
3) Measurement of mycelial growth inhibitory effect of each fractions
Mycelia were placed on PDA containing 10, 20, 50, 100 and 200 ㎍/㎖ of each fractions in six well plates and were incubated for five days. The mycelial growth inhibitory effects were determined by IC50 calculated by measured values of mycelial radii. The experiments were performed in triplicate.
Field test of control rate of rice blast disease
Control rate of A. asphodeloides extract for rice blast disease was determined by Seounmyeon (Ansung, Gyeonggi-Do, Korea) using rice strain of Chucheng strain from the 20th, August to the 19th, September, 2012. Nursery bed test was performed by A. asphodeloides extract, diluted by 500 times and foliage was treated in flood season (2012/8/27, 2012/9/3) at intervals of 7 days. Phytotoxicity test were performed on the 27th, August, 2012 by 250 folds and 500 folds of standard amount. The nursery plants were transplanted by machine on the 23th, 26th, May. Plant spacing was 30 x 14 ㎝ and ear emergence was on the 20th, August. Plant extracts were treated by general use of practices but, other pesticide's sprays were banned. The area of test plot of control rate was 30 ㎡, nursery bed test plot, 10 ㎡ and the area of total test plot was 270 ㎡, respectively. Each test was performed in triplicate by randomized block design (RBD). Nursery bed test was investigated on the degree of diseased panicle after 30 days to heading from 30 rice of each plot. Phytotoxic test was performed by disease assessment on after 3, 5, and 7 days (the 30th, August, the 1th, 3th, September) from treatment day of extract of A. asphodeloides.
Statistical analysis
ANOVA tests using Graphpad Prism were performed for statistical analysis regarding the measured mycelial radii in comparison to each experimental control (treatment with plant extract, pesticide). All experiments were performed in triplicate. P<0.05 is considered statistically significant. In field study of rice plant, Duncan’s multiple range tests (DMRT) using ARM 8 software of Gylling Data Management, INC. were performed for statistical analysis regarding the infected panicle of rice plant in comparison to each experimental control (treatment with plant extract, no treatment). All experiments were performed in triplicate. P<0.05 is considered statistically significant.
Results and Discussion
Mycelial growth inhibitory effect of extract of A. asphodeloides
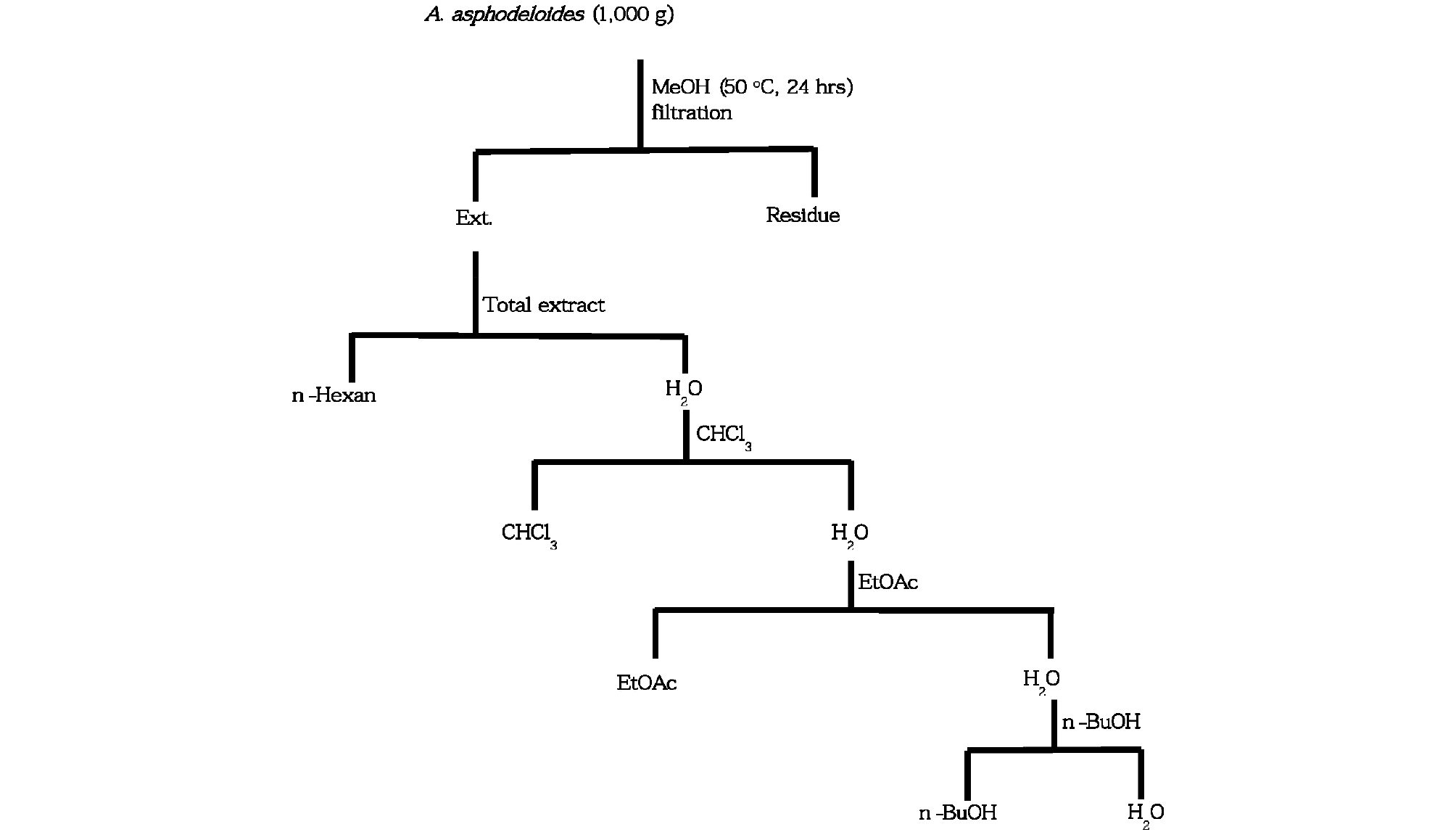
We have investigated possibilities that the extracts of numerous plant species have antifungal activities to mycelial growth of M. oryzae. As we previously reported the inhibitory effect of Lysimachia foenum gracum Herba on the mycelial growth M. oryzae (Lee, 2016), likewise in this study, additional 700 kinds of plant extracts (data not shown) were massively tested for investigation of mycelial growth inhibitory effects about M. oryzae. Among them, A. asphodeloides extract was one of the plant species having the mycelial growth inhibitory effect (Fig. 1). Since the extract showed prominent mycelial growth inhibitory effect on M. oryzae (Fig. 2A), we treated the extract to M. oryzae on PDA media by following concentrations of none treatment, 10, 50, 100, 150, 200, 300, 400, 500, 600 and 700 ㎍/㎖ in dose dependent manner, and determined the IC50, 139.7 ㎍/㎖ based on the measured values of mycelial radii of M. oryzae (Fig. 2B). Comparing the IC50 with the chemical pesticide, tricyclazole, being used for the control of rice blast disease, over the concentration of 300 ㎍/㎖ of tricyclazole are needed to reduce the 24% spore growth of M. oryzae (Froyd et al., 1976). Therefore, the IC50, 139.7 ㎍/㎖ of the total extract is very encouraging value for proceeding further studies because it is needed higher concentration of tricyclazole to inhibit mycelial growth, in this study, than to reduce spore growth in above previous report. From this data, it is possible to suggest that the extract of A. asphodeloides might have a possibility of being a new candidate material of substituting the chemical pesticides used for the control of rice blast disease. So we consider that we found another candidate extract of biopesticide after we previously reported for the case of the extract of L. foenum gracum Herba that had shown the high control rate for rice blast disease (Lee, 2016).
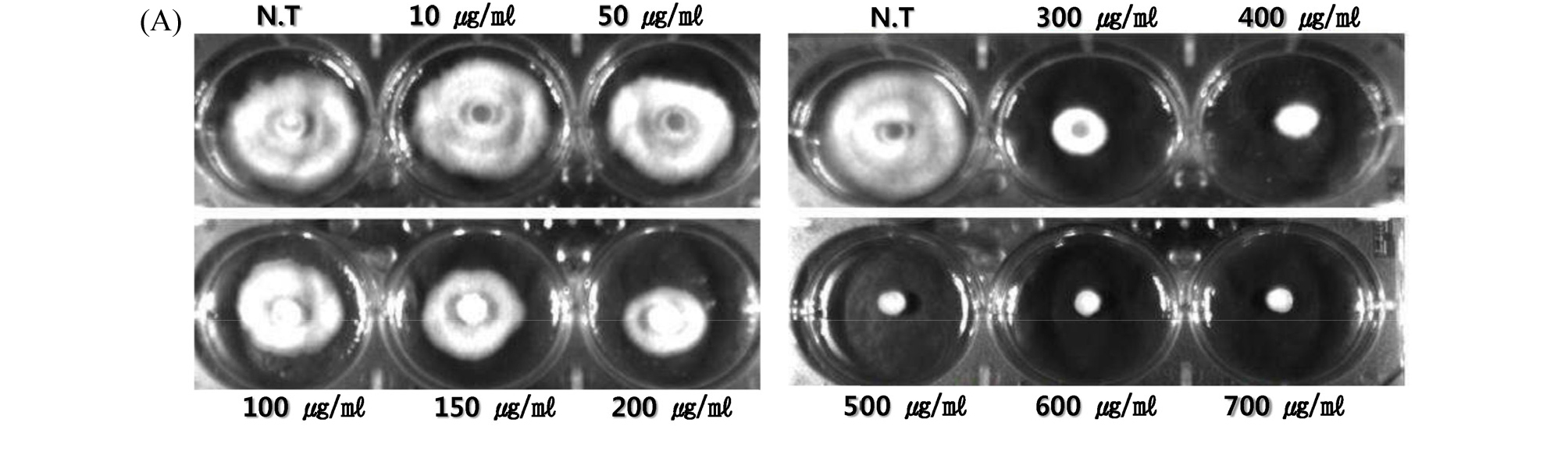
Fig. 2.
Mycelial growth inhibitory effect of extract of A. asphodeloides on M. oryzae in dose dependent treatment. (A) None treatment. Extract was treated by the concentrations of 10, 50, 100, 150, 200, 300, 400, 500, 600, and 700 ㎍/㎖, respectively. Mycelia of M. oryzae cut by cork borer were incubated at 28℃ on the PDA solid media for 5 days. (B) The diameters or radii were determined by mean value of individual measured values. Those experiments were performed in triplicate (p<0.05, Anova test).
Fractions of A. asphodeloides extract have antifungal activities
From above results, it could be possible to suppose that some specific active ingredients from total extract of leaves of A. asphodeloides might have antifungal activities against M. oryzae. So, we prepared fractions of four organic solvents for the total extract, hexan, n-butanol, ethyl acetate, chloroform (Fig. 3) and treated the fractions to mycelia of M. oryzae, respectively, by the concentration of 10, 20, 50, 100, and 200 ㎍/㎖ in dose dependent manner. Among those fractions, ethyl acetate fraction showed most efficient growth inhibitory effects by IC50, 54.12 ㎍/㎖, lower than IC50, 139.7 ㎍/㎖ of total extract A. asphodeloides. (Fig. 4A, 4B). Remarkably, the IC50 value of ethyl acetate is near the level of the IC50, 54.25 ㎍/㎖, of isoprothiolane, a conventional pesticide being used for the control of rice blast disease (data not shown). So, it would be needed to compare the IC50 of ethyl acetate with other conventional pesticides for rice blast disease, presumably supposing that they are comparable enough one another. These results suggest that active ingredients are highly concentrated in ethyl acetate fraction than in total extract, and became possible to explain why the total extract has higher IC50 than that of ethyl acetate. Furthermore, compared to IC50, 139.7 ㎍/㎖ (Fig. 2B), of total extract of A. asphodeloides, the fractions of hexan and chloroform also showed efficiently low enough IC50 of 91.08 and 90.49 ㎍/㎖, respectively, though not to the extent of ethyl acetate fraction. So it is furtherly needed to investigate the possibility of existence of active ingredients in those fractions in the future study. From these results, we could presumably consider that some active ingredients involved in the mycelial inhibitory effects were mainly existed in ethyl acetate fraction, but are also distributed in other fractions.
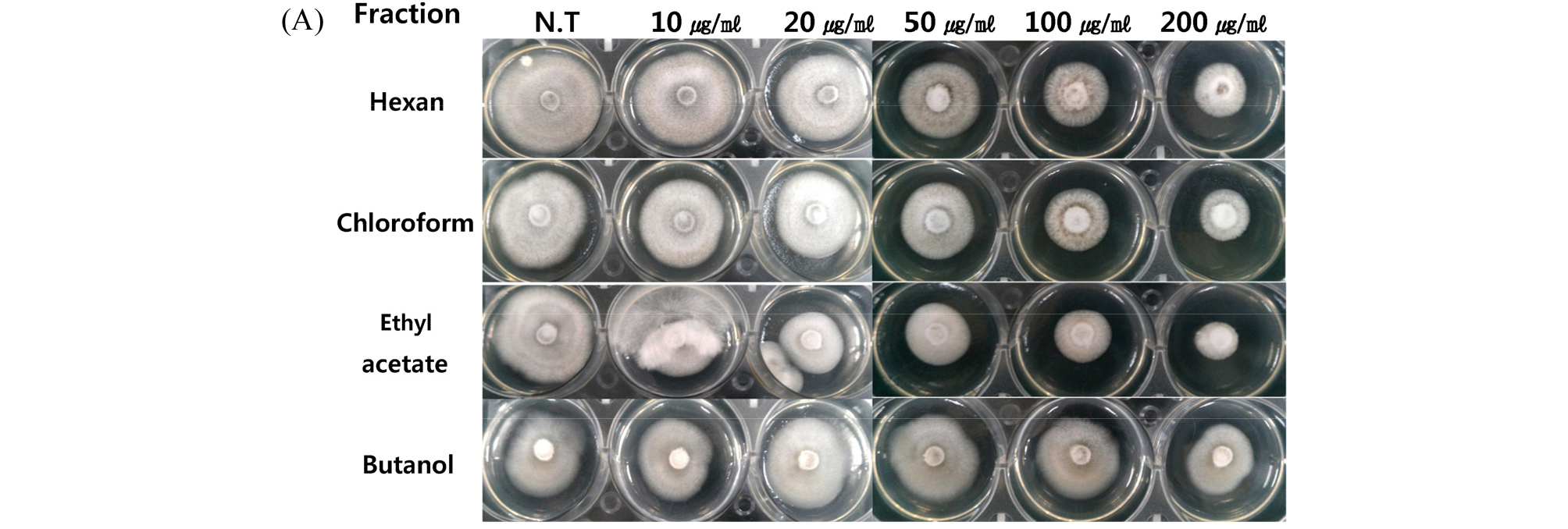
Fig. 4.
Mycelial growth inhibitory effect of fraction. (A) Hexane, Chloroform, Ethyl acetate, and n-butanol fraction were tested by the concentration of 200 ㎍/㎖ of each fraction. (B) Mycelial radii of M. oryzae were measured on the PDA solid media for 5 days after the treatment of ethyl acetate fraction. Those experiments were performed in triplicate (p<0.05, Anova test).
Control effect for rice blast disease of the extract of A. asphodeloides
Since we have studied the mycelial growth inhibitory effect of total extract of A. asphodeloides on M. oryzae, subsequently, we performed the examination for the control rate of total extract A. asphodeloides on rice blast disease in the field of rice cultivation. The completion method was used and the evaluation of standard was performed by comparison to none test port of over 15% of rate of diseased panicle. The rice cultivar. of Chucheong strain was used for its high sensitivity for rice blast disease and two volumes of nitrogenous manure was used more than conventional culture to enhance the occurrence of rice blast disease. Compared to an untreated control plot, we identified 62.0% control rate against rice blast disease (Fig. 5, Table 1). This control rate was confirmed to be statistically significant and the damages to sclerophyll of rice plant was in the standard range and the drainage amount were not found (Fig. 5, Table 2). From these result that total extract A. asphodeloides have very high control rate for the rice blast disease equivalent to that of chemical pesticide for the disease. It could have shown more obvious control rate (>70%) if the total extract were spread before the disease occurrence. It is very meaningful that the extract showed high antifungal activity against M. oryzae in both of the laboratory and crop fields conditions, and we anticipate that ethyl acetate fraction would show higher control rate than that of the total extract of A. asphodeloides.
Fig. 5.
View of test port of rice blast disease field test of rice plant, Chucheng. (A) whole view of testport of none treatment, (B) Symptoms of rice blast disease in none treatment test port by M. oryzae, (C) Whole view of treatment testport, (D) Test port of A. asphodeloides extract treatment. Those experiments were performed in triplicate.
Table 1. Effect of A. asphodeloides extract on control rate of rice in field test
Table 2. Effect of A. asphodeloides extract on phytotoxicity of rice in field test
| Materials |
Testing varieties (Strain) | Phytotoxicity (0~5) | Note | |
| Standard amount | Silution | |||
| A. asphodeloides | Rice (Chucheng) | 0 | 0 | No Phytotoxicity |
In conclusion, we found that the total extract of A. asphodeloides has the mycelial growth inhibitory effect on M. oryzae. So the four different fractions of organic solvents were isolated from the total extract, and identified that ethyl acetate fraction has the highest growth inhibition effect, almost equivalent to that of chemical pesticides. The control rate of the total extract was remarkably, 62.0%, equivalent to that of chemical pesticides, in the rice field for rice blast disease. Finally, it is very meaningful that the extract of A. asphodeloides has high field applicability maintaining high antifungal activity against M. oryzae both of tests, of laboratory and field. Further research would be needed to find active ingredients having antifungal activity leading to the development of new biopesticide and also to verify the cellular mechanisms including the MDR efflux of ABC transporters (Lee et al., 2005; Lee, 2016) concerned with the mode actions between pesticides and the active ingredients of plant extracts.