Introduction
Materials and Methods
DNA extraction, PCR and sequencing
Parent strain and mycelium culture
Establishment of optimal culture conditions
Quantification of adenosine and cordycepin
Statistical Analysis
Results
Isolation and Genetic Characterization of Cordyceps militaris HB8
Optimal culture conditions of Cordyceps militaris HB8
Discussion
Introduction
Cordyceps are Ascomycota, Sordariomycetes, Hypocreales, and Clavicipitaceae mushrooms and there are more than 500 varieties. Currently, it is classified into Cordycipitaceae, Ophiocordycipitaceae, and Clavicipitaceae. Of these, it is known that about 80 species were collected and classified in Korea (Lee et al., 2017). Ophiocordyceps sinensis and Cordyceps militaris are currently widely used in oriental medicine (Shrestha et al., 2012; Tuli et al., 2013). A wild Ophiocordyceps sinensis is very expensive because its specific hosts and conditions are required for growth and is difficult to find it in nature. Cordyceps militaris is a fungus belonging to Cordycipitaceae and Cordyceps that is widely used as a traditional medicinal mushroom in China, Korea, Japan and Asian countries (Sung et al., 2007). However, Cordyceps militaris is gaining widespread attention in the fields of food, medicine and cosmetics as artificial cultivation becomes possible.
Cordycepin (3'-deoxyadenosine) and adenosine, the main physiologically active ingredients in, are analogues of nucleoside, Various functions have been reported, such as immunomodulation (Kim et al., 2012; Lee et al., 2004), antioxidant (Hong et al., 2016), anti-cancer (Park et al., 2002), anti-inflammatory (Choi et al., 2012; Seo et al., 2018), anti-diabetes (Kim et al., 2005), anti-hyperlipidemia (Lee et al., 2006), antithrombosis (Ahn et al., 2012), cognitive function (Cai et al., 2013), anti-viral (Ohta et al., 2007), anti-bacterial (Tran and Tran, 2019), anti-pest (Kim et al., 2002), and skin whitening (Chin et al., 2006). Cordycepin is generally known as a main ingredient in Cordyceps militaris, but the amount contained in wild Cordyceps is less than 0.5% based on dry weight. However, Cordyceps militaris has the advantage of enhancing the production of secondary metabolites from them as artificial culture succeeds in solid medium.
Several researchers have focused on finding the optimal conditions for producing useful ingredients such as cordycepin and adenosine at high concentrations under artificial culture conditions (Cha et al., 2004; Kim et al., 2003). It is known that the production of secondary metabolites from Cordyceps militaris requires specific amino acids, nitrogen sources, carbon sources, minerals, growth enhancer and special culture conditions (Choi et al., 1999).
This study was carried out to investigate the genetic characteristics of the strain Cordyceps militaris HB8 isolated by the authors, and estalish the optimal solid culture conditions to increase the fruiting body yield, contents of cordycepin and adenosin from Cordyceps militaris HB8 based on the nutrient factors and culture environment of the medium.
Materials and Methods
Collecting Cordyceps fungus and investigation of genetic characteristics
The Cordyceps used in this study was a new strain collected from Odaesan, Gangwon-do. The fruiting bodies were washed with sterile water, immersed in 1% sodium hypochlorite (NaClO) solution for 1 minute, and washed again with sterile water thoroughly. The well-washed fruiting bodies were cut into about 5 mm length and cultured in PDA (potato dextrose agar; potato 15 g, glucose 20 g, agar 20 g/L) medium. The newly isolated Cordyceps fungus was named Cordyceps militaris HB8, and the cultured stock culture was subdivided into 10% glycerol solution and stored at -80℃.
DNA extraction, PCR and sequencing
The DNA was extracted by minor modifying the CTAB method of Sung et al. (1999). The rRNA genes in the internal transcribed spacer 1 (ITS1) and (ITS2) regions were amplified using a universal primer (ITS-1:5'-TCCGTAGGTGAACCTGCGG-3'; ITS-4: 5'-TCCTCCGCTTATTGATATGC-3'). PCR conditions were set at 94℃ for 4 minutes, 94℃ for 1 minute and 58℃ for 1 minute (30 cycles), 72℃ for 1 minute, and the final extension at 72℃ for 10 minutes. The PCR products amplified for sequencing of ITS1 and ITS2 regions were sent to Genotech (Daejeon, Korea). The DNA sequence of the analyzed strain HB8 was modified using LASERGENE software (version 6.0; DNASTAR, Madison, WI, USA), and compared with the fungal sequence available in the NCBI database.
The full sequence was compared with BLAST searches on NCBI (https://blast.ncbi.nlm.nih.gov/Blast.cgi). The ITS1/2 gene regions of strain HB8 and closely related strains were complete alignment by using the CLUSTAL_X program (Thompson et al., 1997) and the Kimura two-parameter model (Kimura, 1983). Phylogenetic tree was constructed by the neighbor-joining (Saitou and Nei, 1987), by using the Bioedit (Hall, 1999) and MEGA 5 program (Tamura et al., 2011). Bootstrap analysis based on 1000 replicates was also conducted. The closest strains with the candidate strain were included in the phylogenetic tree construction.
Parent strain and mycelium culture
Cordyceps militaris HB8 was active from stock vial on PDA medium for 10 day and then used for parental culture. The mycelium seed culture was growth in 250 mL conical flask with 100 PDB medium and punching 10 pieces about 0.8 ㎝ was added from active parent culture plate. The flask was incubated at 23℃ with rotating shaking (110 rpm) for 3 days in dark conditions. Large scale culture was grown in a 2000 mL flask containing 1000 mL of a liquid culture solution (10 g of peptone, 1 g of KH2PO4, 0.5 g of K2HPO4, 20 g of glucose, 0.01 g vitamin B1 and 1 g of MgSO4 per L, 100 mL of seed culture was added. The flask was incubated at 23℃ with rotating shaking (110 rpm) for 5 days in dark conditions.
The solid culture was carry out with 30 g of each solid substrate was separately poured into the culture bottle (10 ㎝ diameter, 19 ㎝ height) with 45 mL of basic nutrients medium content: 2 g of yeast extract, 8 g of peptone, 1 g of KH2PO4, 0.5g of K2HPO4, 20g of glucose, and 1 g of MgSO4 per L, and then sterilized at 121℃ for 20 minutes. 10 mL of seed culture was inoculation to each bottle. The culture bottle was transferred to dark condition for 3 days and then transfer to light intensity of 400 to 700 lux condition still 60 day. The light and dark conditions were 14 hours in the light and 10 hours in the dark. At the end of the incubation period, the fruiting bodies of Cordyceps militaris were separated from the solid medium and dried at 40℃. Each experiment was carried out three times.
Establishment of optimal culture conditions
The effect of light
Ten mL of the seed culture solution was inoculated into a sterilized culture bottle containing a solid substrate. The culture bottle was cultured for 3 days under dark room conditions, and then transfer to different light condition: light intensity of 400 to700 lux, 100 lux or less, and 1200 lux or more, respectively. The light and dark conditions were 14 hours in the light and 10 hours in the dark. After 60 days’ culture fruit body was collected and dry at 40℃ for 1 day. The sample was store in -20℃ for further test.
The effect of substrates in the medium
In order to observe the effect of the substrate on the culture for fruiting body of Cordyceps, the effect of the composition of the medium, the type of grain, and the mixing ratio were investigated. That is, The mixing ratio of brown rice (R), millet (M), oats (O) and black beans (B) among the grains in the medium were O : M=25 : 5, R : O=15 : 15, R : O : M = 15 : 10 : 5, and R : O : B : M = 10 : 10 : 5 : 5. 30 g of each solid substrate was separately poured into the culture bottle (10 ㎝ diameter, 19 ㎝ high), 45 mL of nutrients (content: 2 g of yeast extract, 8 g of peptone, 1 g of KH2PO4, 0.5 g of K2HPO4, 20 g of glucose, and 1 g of MgSO4 per L) were added to the culture bottle, and then sterilized at 121℃ for 20 minutes. 10 mL of seed culture was inoculation to each bottle. The culture bottle was transferred to dark condition for 3 days and then transfer to light intensity of 400 to 700 lux condition still 60 days. The light and dark conditions were 14 hours in the light and 10 hours in the dark. Each experiment was carried out in 32 culture bottles. Fruit body was collected and dry at 40℃ for 1 day. The sample was store in -20℃ for further test.
The effect of nitrogen sources
Effect of nitrogen source on the production of cordycepin and adenosine in Cordyceps fruiting bodies, experiments were performed under the same basic medium conditions. Six different nitrogen sources such as yeast extract, soybeans, skim milk powder, egg yolk, fresh pupa, and dry pupa were used. And all experiments were performed triplicate.
The effect of carbon sources
Effect of carbon source on the production of cordycepin and adenosine by Cordyceps cultivation, 20 g/L of different carbon sources such as glucose, sucrose, lactose, maltose and mannose was used as the carbon source in the basic medium, respectively, and performed as a substitute sugar for glucose. The pH of the culture solution was adjusted to 6.0, and the culture conditions were the same under the basic culture conditions, and the experiment was performed triplicate.
The effect of growth factors
To observe the effect of growth factors on the production of cordycepin and adenosine, the fruiting body of Cordyceps, 30 g of rice and 45 mL of nutrient solution (glucose 20 g/L, peptone 10 g/L, MgSO4 1 g/L; KH2PO4 1g/L and 0.01 g/L vitamin B1) as basic culture were mixed to prepare a medium. After dissolving 1 ㎎ each of growth factors 2,4-Chlorophenoxy acetic acid (2,4-D), α-Naphthyl acetic acid (NAA), and Indol-3-butylic acid (IBA) in 1,000 mL distilled water (pH 6.0), it was transferred into plastic bottles and used after autoclaving at 121℃ for 20 minutes.
Quantification of adenosine and cordycepin
Standard materials of cordycepin and adenosine were purchased from Sigma Aldrich, Co., (USA). The series of standard solutions of cordycepin and adenosine were prepared at 12.5, 25.0, 50.0, 75.0, 100.0 and 125 ㎍/mL. Extraction of cordycepin and adenosine from fruiting bodies: 1 g of dried fruiting body powder was suspended in 20 mL of deionized water and sonicated at 42 kHz, 70 W (08890-06, Cole-Parmer, USA) at 60℃ for 1 hour. The supernatant was obtained by centrifugation at 9000 rpm for 10 min.1 mL of supernatant was filtered through a 0.45 μm membrane filter, and then subjected to high performance liquid chromatography (HPLC) analysis.
High performance liquid chromatography (HPLC) analysis was performed on an Aglient infility 1260 HPLC system. The column used was a reversed phase Aglient C18 (4.6 ㎜ × 250 ㎜, 5 μm particle size). Separation of adenosine and cordycepin from the extract was performed using a mobile phase consisting of deionized water and HPLC grade methanol (Merck, Germany) at a column temperature of 25℃ in a relative ratio of 85:15 on a volume basis. The flow rate was 1.0 mL per minute and UV-visible detection was performed at 260 ㎚.
Statistical Analysis
Fresh weight, dry weight, adenosine and cordycepin production are expressed as means ± SD (n=3). An analysis of variance (ANOVA) followed by Tukey’s test was applied for multiple comparisons of significant analyses at P-value < 0.05.
Results
Isolation and Genetic Characterization of Cordyceps militaris HB8
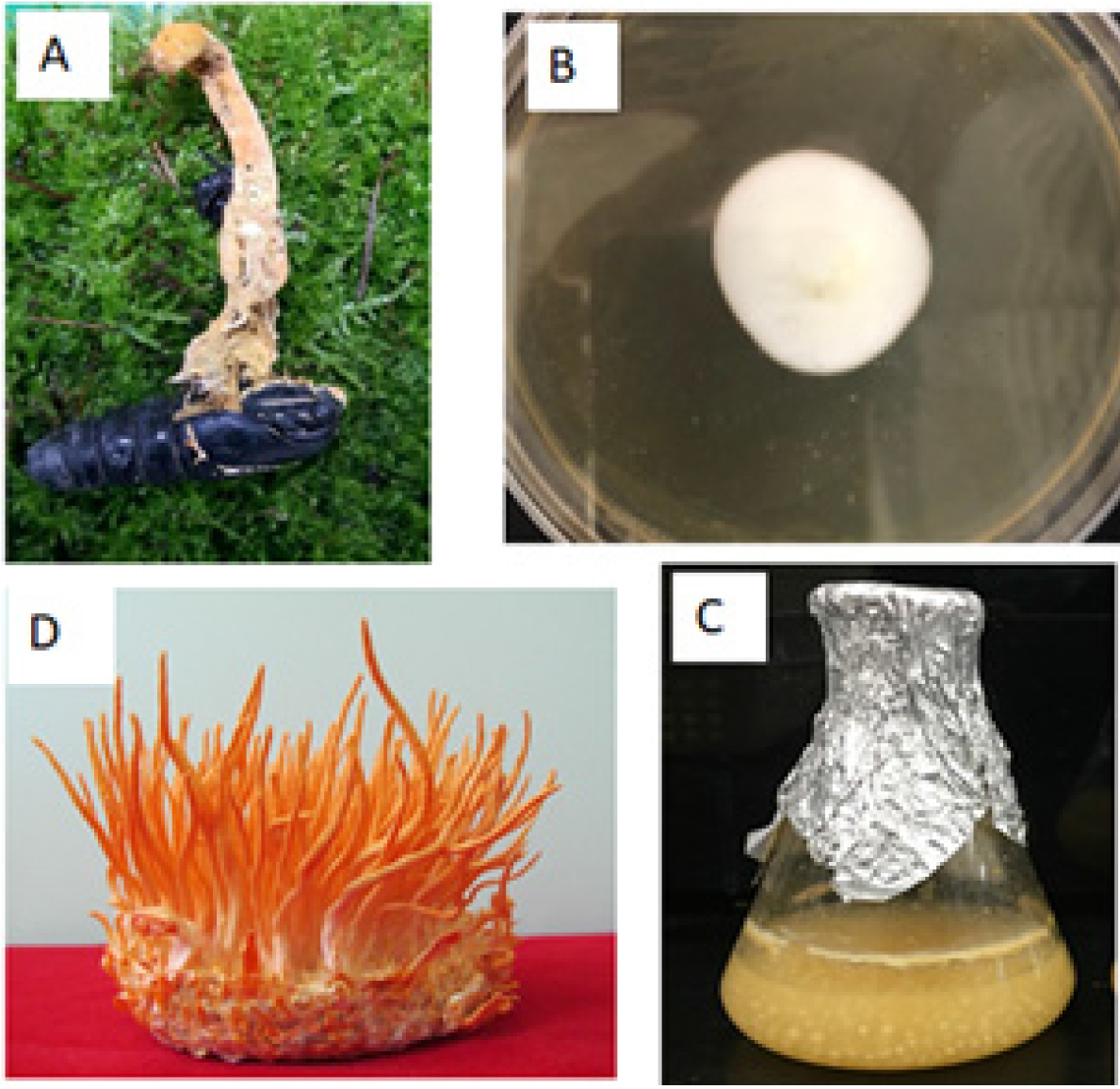
The mycelium on PDA medium of Cordyceps militaris HB8 was isolated from nature specimen was showed on Fig. 1 A ~ C. The result of nucleotide sequence analysis using the universal primer of the internal transcribed spacer (ITS) rDNA of HB8 is published on NCBI with accession number is MT835161. The morphology of fruit body on brown substrate was showed on Fig. 1D.
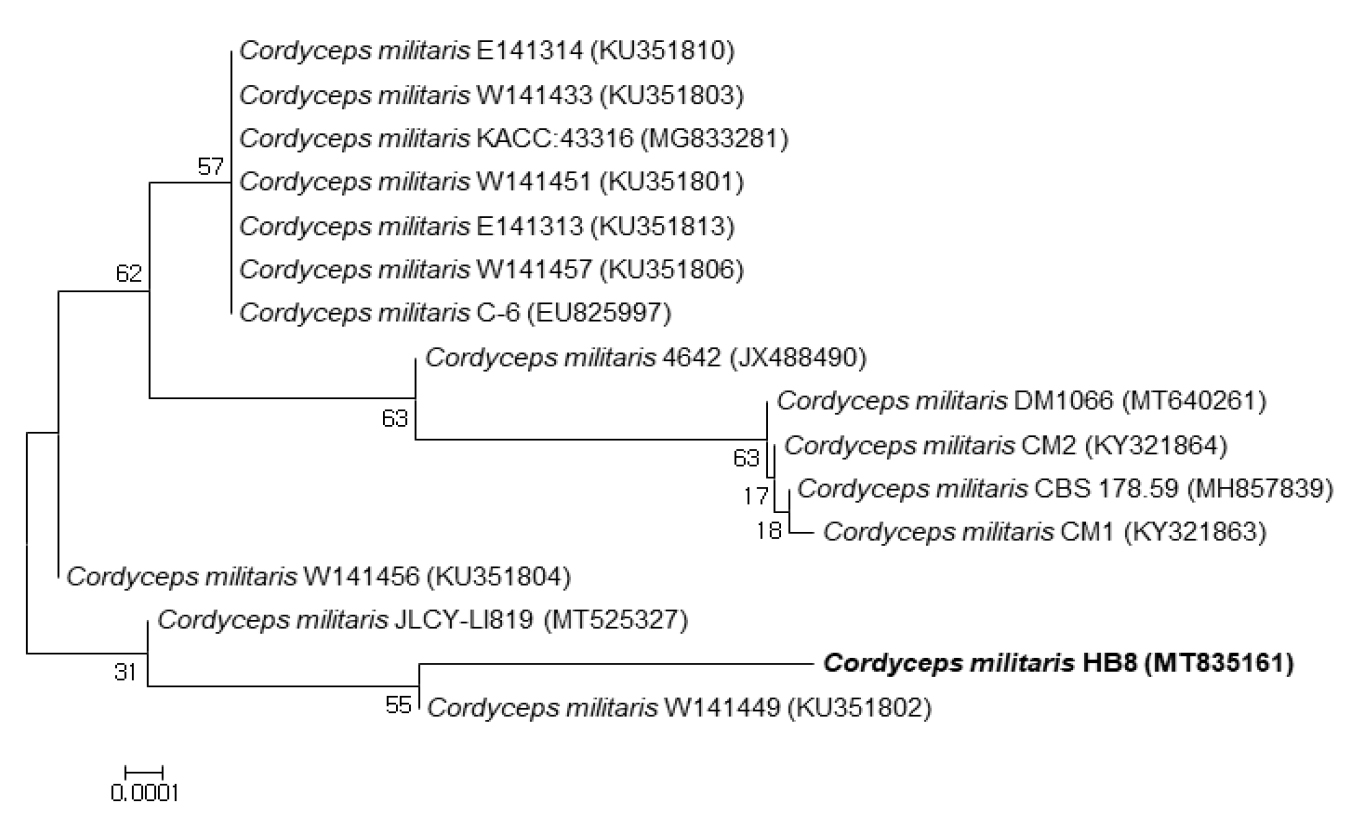
The isolated Cordyceps militaris HB8 (MT835161) was identified as Cordyceps militaris by ITS sequencing. It was most closeted to Cordyceps militaris 4642 (99.81%), Cordyceps militaris JLCY-LI819 (99.82%) and Cordyceps militaris W141456 (99.82%) (Fig. 2).
Optimal culture conditions of Cordyceps militaris HB8
The effect of light
The best light conditions for the growth of Cordyceps militaris HB8 were treatment with 400 to700 lux. In the best conditions, the length of the fruiting body was 7 to14 ㎝, which was longer than 5 to 9 ㎝ at less than 100 lux and 5 to 9 ㎝ at more than 1200 lux (Table 1, Fig. 3). The fresh weight and dry weight of fruiting bodies under light condition 400 to 700 lux was 37.7 g and 6.0 g per bottle, respectively, which was higher than 27.9 g and 4.1 g at 1,200 lux and 24.0 and 3.3 g at 100 lux or less. The adenosine content was the highest at 1,369 ㎍ at less than 100 lux, and the content of cordycepin was 3,586 ㎍ per g of dried fruiting body at 400-700 lux, which was higher than when treated with more than 1,200 lux or less than 100 lux.
Table 1.
Effect of light condition on growth of Cordyceps militaris HB8
The effect of substrates in the medium
In this study, brown rice (R), millet (M), oats (O), black beans (B) were tested by different ratio such as O : M=25 : 5, R : O = 15 : 15, R : O : M = 15 : 10 : 5 and R : O : B : M = 10 : 10 : 5 : 5. Mixture of 15 : 15 ratio with brown rice and oats was found to be a relatively good basic substrate for fruiting growth, The highest yield of fruiting bodies was 44.1 g per bottle. Grains rich in amylopectin, including millet, were not good substrates for the production of fruiting bodies (Table 2). Oats substrate only were showed highest cordyepin production. C. militaris may find it difficult to utilize Amylopectin. The results obtained in our study differ from those using optimum solid substrates to produce C. militaris fruiting bodies and cordycepin which found wheat (Dong et al., 2012).
Table 2.
Effect of different basal substrates on fruiting body, and cordycepin and adenosine production
The effect of carbon sources
Carbohydrates are one of the important elements in cultured cells. Glucose, sucrose, lactose, maltose and mannose were tested to investigate the effect of carbon sources on the production of fruiting bodies and cordycepin in Cordyceps. Glucose medium showed the highest yield and length of fruiting body. Meanwhile, glucose was relatively excellent in the production of adenosine and cordycepin (Table 3). The glucose medium produced the highest fruiting body yield and length of fruit body. Therefore, the use of glucose as a carbon source is considered desirable for the industrial production of Cordyceps fruiting bodies, adenosine and cordycepin.
Table 3.
Effect of carbon sources on the fruiting body, adenosine and cordycepin production by Cordyceps militaris HB8
The effect of nitrogen sources
The results of different nitrogen sources in this study are shown in Table 4. Fresh pupa was best nitrogen source for the length and yield of fruiting bodies production (Fig. 4). On the other hand, skim milk powder was the best nitrogen source for produce adenosine, and the dry pupa was the best source of nitrogen to produce cordycepin. This report was similar with other research on Cordyceps spp. (Kang et al., 2017).
Table 4.
Effect of nitrogen sources on the fruiting body and cordycepin production by Cordyceps militaris HB8
The effect of growth factor
Plant growth factor is one of the important factors in the in vitro culture of cells. In order to find suitable growth factors for growth of fruiting body, production of adenosine and cordycepin, different plant growth factors, namely, NAA, 2,4-D, and IBA were selected and cultured in basal medium after adding each of them. As a result, growth factors did not significantly affect the size and weight of fruiting bodies, and the contents of adenosine and cordycepin. However, only NAA showed somewhat higher yield in the production of fruiting bodies and cordycepin (Table 5).
Table 5.
Effect of different growth factors on fruiting body, adenosine and cordycepin production
Discussion
The efficacy of Cordyceps has been scientifically confirmed in Ophiocordyceps sinensis and Cordyceps militaris belong to the genus of Cordyceps of the Clavicipitaceae family (Das et al., 2010). After the artificial cultivation of the Cordyceps militaris became possible, several researchers isolated new strains and investigated the genetic characteristics (Choi et al., 2009; Jeong et al., 2009; Shrestha et al., 2004). In addition, cultivation of new varieties using hybridization (Lee et al., 2013; Lee et al., 2015). In addition, genetic variation of hybrid lines using RAPD and line selection based on cordycepin content have been reported (Choi et al., 2009; Jeong et al., 2009). In this study, the authors isolated a new Korean native Cordyceps militaris HB8, The ITS, ITS2 gene sequence was showed strain HB8 was closet to Cordyceps militaris W141449 (99.82%), and Cordyceps militaris 4642 (99.81%), and NCBI Asscession number of strain Cordyceps militaris HB8 is MT83516.
In order to increase the production of physiologically active ingredients such as cordycepin in Cordyceps, two major approaches are possible. The first is a method of breeding bacteria with excellent genetic characteristics by selection or hybridization, and the other is a method of controlling and optimizing the culture medium or culture environment. Examples of developing superior bacteria by the former method include a variety called ‘Yedang’ (Lee et al., 2013) and a new variety called ‘Dowon Hongcho’ (Lee et al., 2015). These cultivars were developed as hybrid breeding between them. The content of Cordycepin was 0.31% in Yedang and 0.34% in Dowon Hongcho. This is generally high compared to the level of cordydepin content from nature Cordyceps militaris is 0.05 to 0.15% per dry weight of fruiting body. However, in this study, Cordyceps militaris HB8 collected and isolated from nature had a cordycepin content of 0.8% higher than that of them under optimal conditions. According to the study of Shretha et al. (2004), there are reports that the formation of fruiting bodies becomes unstable in artificial cultivation, so continuous observation of the characteristics of the isolated bacteria is required.
A number of studies were conducted to increase the production of useful components such as cordycepin and fruiting bodies of Cordyceps in the medium composition and culture environment. The important task is to find the most suitable cultivation conditions and environment for the growth characteristics of the fungus. Like other plants, the most important factors in cultivation of Cordyceps are light, temperature, nutrients and pH of the medium. In summarizing the research results, demands vary greatly depending on the type of bacteria. That is, the pH is 5 to 7, the temperature is 10 to 25℃, the illuminance (300 to 1,200lux) and the light source, medium (MCM, PDA, SDAY, YM, MMM), trace component requirements, and culture time are very different (Choi et al., 2009; Hur, 2008; Jo et al., 2004, 2005; Lee et al., 2015).
In this study, the temperature and pH were similar to the results of other researchers at 23℃ and around 6.5, but there were differences in the composition of the medium. In other words, in the case of substrate, the mixed medium of brown rice and oats was the best in this study, but it was different from the results of Dong et al. (2012) that wheat was good. In the carbon source, glucose was the best, as shown by Kang et al. (2017). However, through this study, it was found that amylopectin is not preferable as a carbon source.
In particular, it was confirmed that the selection and mixing ratio of the substrate not only had a significant effect on the sex of cordycepin or adenosine, but also differed according to the isolated bacteria (Cha et al., 2004; Dong et al., 2012; Jo et al., 2005; Kim et al., 2014). On the other hand, it is a peculiar fact that the growth-promoting factor did not significantly affect the growth of this fungus compared to other factors. In other words, in this study, when cultured around 500 lux, the production of fruiting bodies was the highest, which was about 50% higher than those under 100 lux or 1,200 or more. In addition, pink light has been reported to increase the production of cordycepin (Dong et al., 2012), and it was found that illuminance is an important factor in the production of specific components in cells during the growth of Cordyceps miltaris bacteria (Dong et al., 2012; Jo et al., 2005).
Since Cordycepin and adenosine are substances in the same metabolic line, it is not easy to enhance the two components simultaneously according to metabolic flow. As described above, the medium requirements and culture conditions for increasing the components of cordycepin and adenosine were slightly different. Nevertheless, it was found that supplying fresh pupae as a nitrogen source could slightly increase both adenosine and cordycepin components at the same time. Therefore, in order to increase the content of specific components in Cordyceps militaris, it is thought that it is necessary to understand the characteristics of individual bacteria and to select an appropriate medium and environment.
The most well-known medicinal ingredient in Cordyceps militaris is cordycepin, an adenosine analogue. Cordycepin is a nucleic acid material, similar to the structure of deoxyadenosine. It engages in the genetic information of cells, activates the reduced immune function, and prevents normal cells from mutating into cancer cells. Therefore, cordycepin is a representative substance that plays a role in enhancing immune function and suppressing cancer. As described in the introduction, it also has various functions such as anti-inflammatory, antibacterial, and navigating insect action (Chin et al., 2006; Kim et al., 2002; Kim et al., 2012; Lee et al., 2009; Lee et al., 2004).
The second well-known ingredient is adenosine. Adenosine is a purine nucleoside in which adenine, a nucleobase, and ribose, a pentose, are linked by a β-N9-glycoside bond. Derivatives of adenosine are widely found in nature and play an important role in biochemical processes such as energy transfer as well as signaling. Adenosine is also a neuromodulator, promotes sleep, inhibits arousal, and regulates blood flow in various organs through vasodilation (Masino et al., 2011). Adenosine is a substance recognized by the Ministry of Food and Drug Safety as a raw material for functional cosmetics for improving wrinkles. Adenosine exhibits excellent efficacy in improving cell regeneration and enhancing skin elasticity by synthesizing proteins inside, not the epidermis (Yang et al., 2019). In addition, it is a natural raw material that can be used regardless of night or day, unlike retinol, a well-known wrinkle-improving functional substance, because it has no toxicity and side effects and has strong lasting power. Therefore, more than 90% of the anti-wrinkle functional cosmetics products currently distributed in Korea use adenosine as a functional ingredient. In addition, it contains various useful ingredients such as ergosterol and vitamin C, which are vitamin D precursors (Nallathamby et al., 2015).
As the usefulness of Cordyceps has been revealed, a technique that can be produced and separated in large quantities by searching for strains containing high specific components such as cordycepine or changing culture conditions has been continuously reported (Lou et al., 2019; Tran and Tran, 2019; Zhang et al., 2019). Cordyceps militaris HB8, newly isolated in this study, is considered to be applicable as a strain containing high levels of cordycepin or adenosine.