Introduction
Materials and Methods
Preparation of plant samples
Reagents and chemicals
Extraction of β-carotene
Analysis of β-carotene
Cucumisin activity assay
Statistical analysis
Results and Discussion
Fruit morphology analysis
Analysis of β-carotene and cucumisin using spectrophotometric and HPLC methods Method validation
Introduction
Melon (Cucumis melo L.) belongs to the fruit Cucurbitae family is one of the most cultivated fruits in tropical countries, and a good source of essential vitamins, ascorbic acid, including pro-vitamine A and folic acid (Xudong et al., 2020). Melon represents the variability of expressive and biochemical properties based on region and climate (Hari and Prashant, 2021; Yan et al., 2006), and has a wide range of morphological colors, shapes, flavors and textures. Melon has a variety of biological properties, such as cardiovascular disease, anti-cancer, anti-inflammatory, antioxidant, and is used in the treatment of diarrhea, diuretics and pain, and consumption is increasing (Awika et al., 2003; Ricardo et al., 2020, 2021; Rodríguez et al., 2013; Saini et al., 2015; Shofian et al., 2011).
Melon flesh is rich in carotenoids including α-carotene, β-carotene, fatty acids including polyphenols, linoleic acids, oleic acids including phenolic acids and flavonoids (Ik et al., 2021; Lester, 2008). Cucumisin is an alkaline serine protein enzyme in the pulp that promotes digestion and helps proteins become an efficient source of energy (Asif-Ullah et al., 2006; Yoo et al., 2020). β-Carotene is an antioxidant, a major precursor of vitamin A (Peinado et al., 2016; Yemesrach et al., 2021), and a powerful iron enhancer (Butler and Ghugre, 2020; Richard and Ines, 2010). It is also reported to perform a variety of biological activities such as increased immune response, treatment of cardiovascular diseases, anti-inflammatory and anti-cancer activities (Li et al., 2019; Lingyu et al., 2021; Wahyono et al., 2019). Recently, as interest in including fresh fruits in the human diet has increased, high quality and production have been demanded to ensure sufficient food supply (Claire et al., 2007; Melisa et al., 2016).
The purpose of this study is provide basic data for high functional varieties development studies by conducting comparative analysis of morphological characters, β-carotene content and cucumisin activity using 58 melons selecting highly useful germplasm resources.
Materials and Methods
Preparation of plant samples
Seeds of 58 melon genetic resources (57 germplasm collections and 1 commercial varieties) those were from originated from 38+ countries were obtained from the gene bank of the National Agrobiodiversity Center (NAC), Rural Development Administration (RDA), Jeonju, South Korea. The seeds were sown at the research farm of the NAC. RDA’s recommended cultural management practices for melon were followed in the experimental field. Fertilizers were applied (N-P2O5-K2O = 13.8-4.9-8.7 ㎏/10a) followed by RDA’s standard and drip irrigation tape was used for watering. Seeds were sowed on March 27, 2020, and grown in a nursery bed for 30 days. Seedlings (twelve plants from each accession) were transplanted at an area of 45 ㎝ × 30 ㎝ in a polyethylene vinyl greenhouse equipped with insect net to prevent insect pollination. Plants of the same accession were grown in a single plot (plot area 12.6 m2). They were pollinated by hand and harvested after 60 days (on average) of pollination. Melon fruits were harvested at a fully mature stage, collected, stored in polyethylene bags, and immediately transferred to a -18℃ walk-in freezer until further processing. The flesh (mesocarp) of the melon fruit was carefully separated from the seeds and rind manually and the edible part was, juiced, frozen at -80℃, and lyophilized using vacuum freeze drier (Ilishibiobase, Rijssen, Netherlands). Lyophilized powdered samples were sealed to prevent moisture absorption and stored at -20℃ until analysis.
Reagents and chemicals
β-Carotene as a certified reference material of (all trans form), butylated hydroxytoluene (BHT), ρ-nitroaniline (pNA) and dimethyl sulfoxide (DMSO) were purchased from Sigma- Aldrich Corp. (St. Louis, MO, USA). Glt-Ala-Ala-Pro-Leu- pNA for cucumisin assay was purchased from Peptide Institute, Inc (Osaka, Japan). HPLC-grade acetone, ethanol and n-hexane were from J. T. Baker (Avantor Performance Materials, LLC., Center Valley PA, USA). Other reagents were of analytical grade from commercial sources (Daihan Scientific Co., Ltd. Wonju, Gangwon-do, Republic of Korea).
Extraction of β-carotene
Extraction of β-carotene was performed by low volume n- hexane extractiom method as in previous reported methods (Davis et al., 2008-2009; Fish et al., 2002) with some modification. Briefly, 0.2 g (determined to the nearest 0.001 g) were weighed from the powdered samples into a 50 mL cornical tube containing 3 mL distilled water and thereafter shaken untill completely dissolved with vigorous shaking (about 3 minutes). After shaking, 20 mL of solvent mixture containing 0.05% in BHT in acetone, 95% ethanol and n-hexane 1:1:2 (v/v/v) were added in the tube. The mixture were extracted on an orbital shaker at 120 rpm for 15 min in a dark. The solution were left at room temperature for 5 min to allow for phase seperation and then the supernatants were filtered using a 0.45 ㎛ microsyringe filter. All steps from sample preparation to extraction were performed in subdued lighting condition. The upper layer (n-hexane layer) containing β-carotene was used for HPLC and spectrophotometric analysis.
Analysis of β-carotene
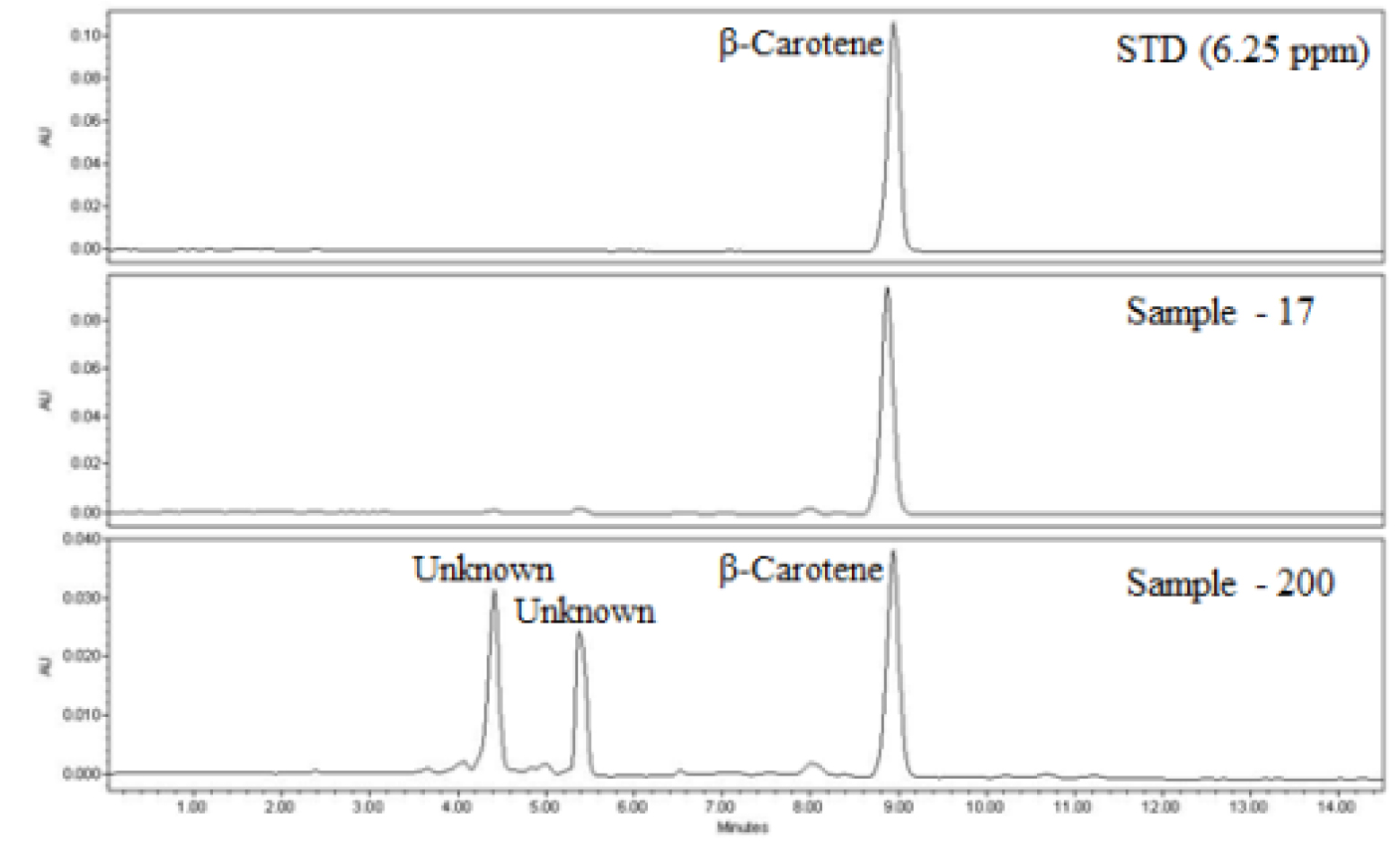
HPLC analysis of β-carotene was done using Waters HPLC system equipped with a 2690 separation module and Waters 996 diode array detector (Milford, MA, USA). The HPLC separation was conducted on Waters Sunfire C18 column (4.6 × 250 ㎜, ID 5 ㎛) with a Sunfire C18 guard cartridge (4.6 × 20 ㎜, 5 ㎛) using a linear gradient mode and a flow rate of 1.0 mL/min. The mobile phase consisted of acetone (A) and deionized water (B) using the elution gradient 85 - 95% B at 0 - 2 min, 95 - 100% at 2 - 11 min and 100% - 85% A at 11 - 15 min. Detection wavelength was set at 479 ㎛ and injection volume was 30 µL. Results were expressed in milligram/ kilogram (㎎/㎏) on a dry weight basis and calculated using an external calibration curve equation (Y = 422537X + 59538 R2 = 0.9996; Y = peak area, X = β-carotene standard concentration) prepared using serial dilutions (0.195 - 25.0 ㎍/mL) of β- carotene standard. β-carotene was eluted at a retention time (tR) of 9.0 minutes (Fig. 1).
Spectrophotometric determination of β-carotene was done using a Shimadzu UV-1601 UV-Visible (UV) spectrophotometer (Kyoto, Japan) and VersaMax tunable microplate reader (MR) spectrophotometer (Molecular Devices, California, USA), respectively. The absorbance of β-carotene by UV and MR spectrophotometers were measured against hexane (blank) at 479 nm, respectively and results were calculated based on an external calibration curve constructed from serial dilutions of β-carotene concentration. The calibration curves were Y = 0.2462X + 0.0211 (R2 = 0.9993) for UV spectrophotometer and Y = 0.422537X + 0.0532 (R2= 0.9991) for MR spectrophotometer, respectively where Y is absorbance and X is concentration of β-carotene standard. Final results were expressed as ㎎/㎏ on a dry weight basis.
Cucumisin activity assay
Extracts for cucumisin assay were prepared by dissolving 0.10 g of the freeze-dried powdered samples of flesh melon using 5 mL 0.1 M sodium phosphate buffer solution (pH 7.5) in a 15 mL cornical tube with screw cap. The solution was vortexed fro about 10 seconds and thereafter shaked for 10 min using orbital shaker (120 rpm), centrifuged (4500 rpm, 10 min). The supernatents were filtered using a 0.45 ㎛ microsyringe filter. The filtrates held at 4℃ untill use. Cucumisin activity was assayed using Glt-Ala-Ala-Pro-Leu-pNA (16 ㎎ in 2 mL DMSO) as a substrate according to the previously reported methods with some modification (Nakagawa et al., 2010; Rudenskaya et al., 1995; Uchikoba et al., 2000). The reaction consisted of 100 µL of sample extract and 0.1 M sodium phosphate buffer (pH 7.4) 1.5 mL was preincubated at 37℃ for 1 min and then added 50 µL of a substrate solution. After incubating for 10 min at 37℃, the reaction was terminated by adding 350 µL of 50% acetic acid. The amount of ρ-nitroanilline released was measured at 405 ㎛ using UV-Vis spectrophotometer and microplate reader, rescprctively. One unit of enzyme acticity was defined as the amount that liberates 1 μmole of ρ-nitroanilline per min under the above-described conditions.
The amount of cucumisin activity in the freeze-dried sample was based on a calibration curve constructed from serially diluted solutions of ρ-nitroanilline concentration and the calculated as follows.
Abs = absorbance; CC = μmole of p-nitroaniline calculated by calibration curve; DF = dilution factor; SV = volume of sample dissolving solution (mL); S = volume of sample for react (mL); T = react time (minute); Sq = amount of powder sample (㎎); 2 = volume of react solution (mL)
Statistical analysis
Each value was expressed as the mean ± standard deviation (SD) of three independent experiments. Quantitative morphological characters were reported as averages of values from 10 to 12 melon fruits. Correlation analysis was conducted using SPSS V25 statistical program (Version 4.0.2, R Studio, Inc.,). PCA analysis was conducted using R-program (Version 4.0.2, R Studio, Inc.,).
Results and Discussion
Fruit morphology analysis
Based on the International Union for the Protection of New Varieties of Plants (UPOV) for melon fruits (International Union for the Protection of New Varieties of Plants (UPOV, 2019), 10 phenotypic characters of melon fruit were assessed. The shape of fruit (SF), the color of flesh (CF), width of fruit (WF), strength of fruit flesh (SFF), color of fruit skin (CFS), the length of seed (LS) and width of seed (WS) were measured with the help of a meter digital balance as required, and other qualitative characters were recorded on the field and inside the laboratory. β-carotene of melon resources contained an average of 48.76 ㎎/㎏, with a content range of 0.37-188.95 ㎎/㎏. The control resource was 129.2 ㎎/㎏. The β-carotene high content resource was S/No.44 (K190274 (188.95 ㎎/㎏)), which was about 1.5 times higher than that of the control resource (Table 1).
Table 1.
β-carotene and cucumisin content and some selected quantitative morphological characters in melon fruit flesh samples
|
S/ No | LLz (㎝) | LJy (㎝) | WFx (㎝) | LSv (㎝) | WSu (㎝) | β-carotene content (㎎/㎏) | cucumisin (U/㎎) | |||
| UV | MR | HPLC | UV | MR | ||||||
| con1 | 21.7 | 12.8 | 14.4 | 1.0 | 0.5 | 155.3±2.19 | 149.6±1.16 | 129.2±4.54 | 0.50±0.01 | 0.47±0.03 |
| 1 | 22.2 | 12.5 | 16.1 | 0.8 | 0.4 | 128.8±2.88 | 118.64±3.57 | 103.47±1.51 | 1.01±0.03 | 1.01±0.06 |
| 2 | 19.2 | 7.5 | 14.4 | 1.5 | 0.4 | 134.82±2.68 | 127.39±5.2 | 113.33±1.71 | 0.78±0.01 | 0.76±0.03 |
| 3 | 21.3 | 10.3 | 15.9 | 0.9 | 0.5 | 56.09±0.42 | 48.98±0.97 | 39.81±0.92 | 1.00±0.02 | 0.99±0.01 |
| 4 | 23.8 | 11.7 | 14.3 | 1.0 | 0.4 | 195.62±2.95 | 182.38±2.71 | 152.42±1.57 | 0.89±0.00 | 0.74±0.04 |
| 5 | 13.0 | 10.0 | 15.0 | 1.0 | 0.5 | 59.95±0.65 | 54.47±2.25 | 51.68±1.54 | 1.96±0.02 | 1.89±0.02 |
| 6 | 18.0 | 11.0 | 9.3 | 0.8 | 0.4 | 151.34±1.3 | 152.74±1 | 119.45±1.59 | 0.35±0.01 | 0.33±0.01 |
| 7 | 20.7 | 12.3 | 14.0 | 1.0 | 0.4 | 64.83±1.22 | 57.35±3.15 | 46.2±1.36 | 2.82±0.12 | 2.70±0.00 |
| 8 | 20.9 | 21.3 | 17.5 | 0.9 | 0.4 | 18.93±0.31 | 18.75±0.5 | 10.15±0.18 | 1.40±0.03 | 1.34±0.01 |
| 9 | 24.6 | 11.5 | 9.4 | 0.9 | 0.4 | 17.24±0.6 | 16.19±0.68 | 6.2±0.11 | 2.40±0.12 | 2.19±0.07 |
| 10 | 6.1 | 7.9 | 4.8 | 0.4 | 0.2 | 17.91±2.45 | 20.26±4.52 | 6.52±0.83 | 7.96±0.05 | 7.76±0.12 |
| 11 | 25.3 | 11.4 | 16.0 | 1.2 | 0.4 | 52.3±1.35 | 50.66±1 | 38.11±1.63 | 1.78±0.00 | 1.73±0.02 |
| 12 | 21.0 | 11.0 | 14.8 | 1.0 | 0.4 | 88.11±0.73 | 82.21±0.53 | 64.74±0.83 | 3.13±0.1 | 3.13±0.06 |
| 13 | 23.3 | 13.0 | 14.2 | 1.0 | 0.5 | 8.57±0.31 | 6.88±0.99 | 1.71±0.23 | 1.76±0.12 | 1.80±0.12 |
| 14 | 14.6 | 8.5 | 10.4 | 0.8 | 0.4 | 72±1.35 | 65.34±2.2 | 53.02±2.46 | 0.96±0.02 | 0.90±0.02 |
| 15 | 19.7 | 11.0 | 13.5 | 1.1 | 0.5 | 14.94±0.51 | 19.57±3.36 | 8.57±0.23 | 1.48±0.11 | 1.39±0.06 |
| 16 | 17.3 | 5.0 | 7.9 | 0.7 | 0.4 | 20.35±0.23 | 22.52±0.79 | 13.42±0.11 | 0.83±0.01 | 0.83±0.00 |
| 17 | 21.8 | 10.5 | 10.6 | 1.0 | 0.4 | 46.14±39.12 | 53±2.44 | 43.48±1.6 | 2.20±0.10 | 2.20±0.11 |
| 18 | 21.0 | 15.5 | 11.6 | 1.0 | 0.4 | 6.88±0.35 | 6.98±1.09 | 3.32±0.13 | 0.42±0.02 | 0.55±0.20 |
| 20 | 22.8 | 12.0 | 13.3 | 1.0 | 0.5 | 7.29±0.6 | 3.36±0.5 | 0.37±0.36 | 2.69±0.09 | 2.52±0.03 |
| 21 | 18.7 | 10.2 | 12.2 | 1.3 | 0.5 | 84.32±1.72 | 77.62±0.95 | 62.92±1.94 | 1.58±0.06 | 1.60±0.03 |
| 22 | 18.0 | 10.2 | 14.5 | 1.0 | 0.5 | 55.35±0.93 | 52.24±1.55 | 40.24±1.02 | 0.98±0.01 | 0.97±0.01 |
| 23 | 20.3 | 8.2 | 13.4 | 0.9 | 0.4 | 154.73±7.31 | 142.35±7.12 | 124.89±9.45 | 0.88±0.02 | 0.85±0.02 |
| 24 | 22.0 | 12.8 | 14.4 | 1.0 | 0.4 | 13.45±0.31 | 16.1±0.14 | 7.39±0.31 | 1.67±0.01 | 1.62±0.00 |
| 25 | 14.5 | 9.0 | 9.1 | 0.6 | 0.3 | 11.89±0.71 | 11.52±1.46 | 6.44±0.31 | 1.02±0.08 | 1.00±0.02 |
| 26 | 21.4 | 11.5 | 14.5 | 1.1 | 0.5 | 63.95±1.07 | 60.08±2.19 | 46.36±1.59 | 0.30±0.02 | 0.31±0.02 |
| 27 | 14.8 | 9.5 | 7.2 | 1.1 | 0.4 | 127.78±0.82 | 120.46±2.7 | 65.57±0.36 | 0.64±0.03 | 0.62±0.03 |
| 28 | 25.2 | 9.7 | 13.7 | 1.0 | 0.5 | 178.01±3.76 | 166.64±2.66 | 145.72±1.9 | 1.84±0.06 | 1.80±0.01 |
| 30 | 26.6 | 11.5 | 11.8 | 1.1 | 0.5 | 86.35±0.42 | 77.17±0.41 | 61.42±0.76 | 0.33±0.04 | 0.38±0.02 |
| 31 | 23.0 | 11.7 | 7.3 | 1.0 | 0.5 | 17.91±0.65 | 23.18±1.09 | 10.54±0.24 | 3.60±0.04 | 3.48±0.00 |
| 32 | 19.6 | 10.5 | 15.0 | 1.0 | 1.4 | 11.42±0.42 | 9.43±1.04 | 3.72±0.54 | 0.57±0.02 | 0.69±0.01 |
| 33 | 18.2 | 10.3 | 15.6 | 1.0 | 0.5 | 147.01±1.01 | 136.84±4.87 | 115.66±0.47 | 0.35±0;00 | 0.39±0.02 |
| 34 | 18.3 | 11.3 | 37.8 | 1.0 | 0.4 | 23.06±0.23 | 18.85±1.63 | 14.01±0.11 | 2.31±0.07 | 2.21±0.07 |
| 35 | 21.5 | 11.7 | 8.9 | 1.3 | 0.5 | 39.92±1.52 | 35.65±2.16 | 22.81±0.47 | 2.32±0.06 | 2.18±0.07 |
| 36 | 17.8 | 8.2 | 7.5 | 0.8 | 0.4 | 98.95±2.57 | 95.54±1.6 | 39.61±0.29 | 1.23±0.06 | 1.19±0.03 |
| 37 | 21.8 | 10.5 | 15.4 | 1.2 | 0.5 | 37.55±0.6 | 32.27±1.33 | 16.62±0.31 | 1.41±0.05 | 1.43±0.06 |
| 38 | 27.8 | 14.0 | 16.2 | 1.4 | 0.5 | 226.15±4.81 | 220.92±6.51 | 180.15±3.1 | 1.16±0.02 | 1.15±0.01 |
| 39 | 22.3 | 13.8 | 14.0 | 1.3 | 0.4 | 77.49±0.58 | 71.13±1.83 | 56.3±0.76 | 0.65±0.02 | 0.64±0.03 |
| 40 | 15.7 | 12.2 | 9.5 | 1.2 | 0.5 | 156.22±1.77 | 145.13±5.95 | 108.25±0.36 | 1.24±0.02 | 1.20±0.01 |
| 41 | 12.3 | 13.2 | 10.4 | 1.0 | 0.5 | 21.03±0.51 | 20.3±2.52 | 10.66±0.18 | 2.26±0.07 | 2.19±0.02 |
| 42 | 9.7 | 6.7 | 7.5 | 0.9 | 0.4 | 198.86±8.59 | 175.57±12.25 | 108.28±5.34 | 1.35±0.05 | 1.33±0.06 |
| 43 | 15.5 | 11.7 | 11.3 | 1.1 | 0.5 | 93.46±1.3 | 89.29±2.55 | 66.79±1.82 | 0.73±0.01 | 0.68±0.01 |
| 44 | 18.3 | 12.0 | 12.2 | 1.0 | 0.5 | 233.59±1.84 | 226.45±4.56 | 188.95±1.87 | 0.91±0.01 | 0.91±0.03 |
| 45 | 23.5 | 12.0 | 12.5 | 1.2 | 0.5 | 16.29±0.71 | 15.19±0.32 | 8.89±0.06 | 1.09±0.05 | 1.05±0.02 |
| 46 | 12.8 | 10.0 | 8.4 | 1.2 | 0.5 | 12.77±0.88 | 14.54±1.31 | 2.06±0.23 | 1.61±0.00 | 1.51±0.04 |
| 47 | 17.3 | 12.0 | 8.6 | 0.7 | 0.3 | ND | 16.34±0.85 | 11.09±0.24 | 0.51±0.01 | 0.50±0.00 |
| 48 | 12.5 | 10.3 | 10.0 | 1.0 | 0.4 | 226.96±6.64 | 205.82±2.66 | 187.53±0.69 | 0.40±0.01 | 0.39±0.01 |
| 49 | 20.0 | 13.3 | 13.1 | 1.1 | 0.5 | 4.31±1.32 | 9.43±1.01 | 2.61±0.18 | 1.92±0.08 | 1.92±0.05 |
| 50 | 14.7 | 13.7 | 8.0 | 0.6 | 0.3 | 18.79±0.82 | 25.21±0.69 | 7.23±0.06 | 0.95±0.03 | 0.9±0.02 |
| 51 | 16.8 | 8.3 | 8.7 | 0.7 | 0.3 | 153.98±0.91 | 154.59±0.87 | 110.34±1.45 | 0.46±0.03 | 0.44±0.01 |
| 52 | 17.8 | 13.0 | 15.8 | 0.9 | 0.4 | ND | 8.03±1.67 | 2.81±0.18 | 0.42±0.02 | 0.49±0.01 |
| 53 | 22.2 | 11.7 | 10.5 | 1.2 | 0.5 | 23.06±0.91 | 17.00±0.5 | 0.88±0.11 | 1.29±0.01 | 1.25±0.02 |
| 54 | 17.8 | 20.7 | 11.0 | 1.0 | 0.4 | 21.91±1.21 | 15.64±1.54 | 7.47±0.44 | 3.7±0.07 | 3.52±0.07 |
| 55 | 14.3 | 8.8 | 9.0 | 0.6 | 0.3 | 31.99±0.84 | 26.69±1.86 | 9.91±0.18 | 0.42±0.01 | 0.47±0.01 |
| 56 | 16.0 | 7.3 | 54.0 | 0.6 | 0.3 | 86.22±2.85 | 80.4±3.77 | 63.63±0.90 | 0.44±0.01 | 0.42±0.01 |
| 57 | 21.2 | 9.8 | 5.8 | 1.2 | 0.5 | 37.48±1.44 | 31.87±0.77 | 20.48±0.24 | 1.61±0.02 | 1.50±0.01 |
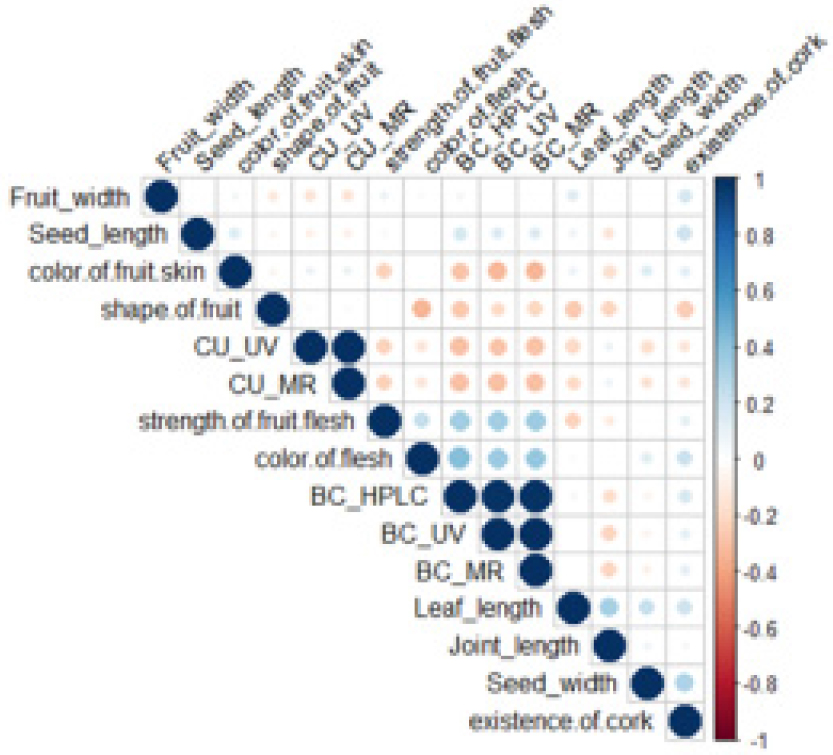
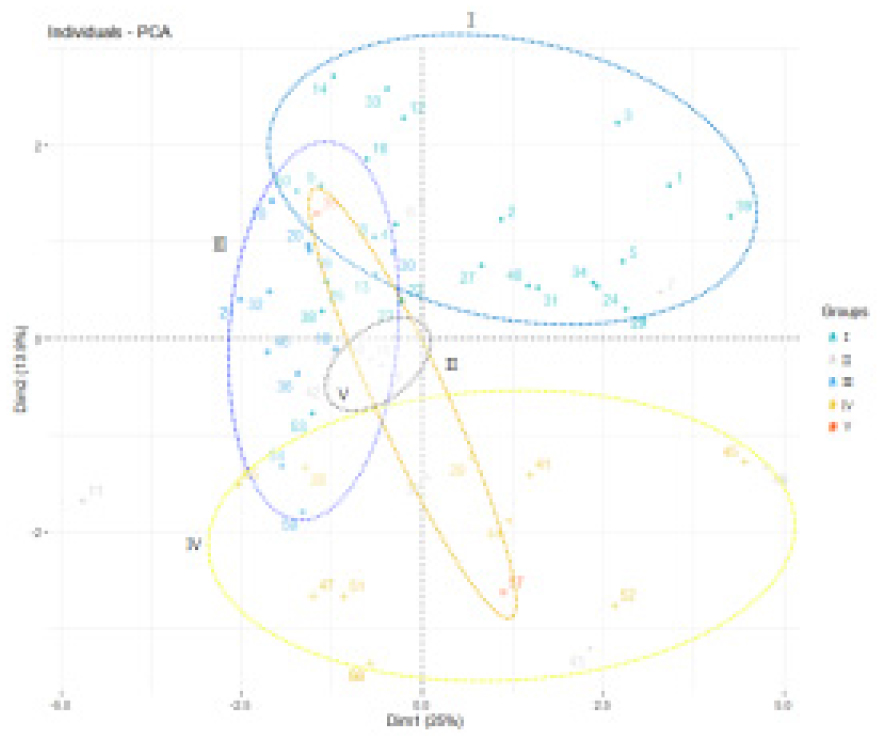
The strength of fruit flesh and color of flesh have positive correlation coefficients with β-carotene (HPLC, UV, and MR). The length of leaf had a positive correlation with the length of joint. The width of seed had a positive correlation with the existence of cork (Fig. 2). Morphological characters, β-carotene content, and cucumisin of five groups of melon fruits were determined. β-carotene content in groups Ⅰ, Ⅱ, and Ⅲ, Ⅳ, and Ⅴ were 82.34±43.34 ㎎/㎏, 86.75±64.99 ㎎/㎏, 25.56±2.09 ㎎/㎏, 86.25±61.7 ㎎/㎏, and 54.56±19.91 ㎎/㎏ respectively (Fig. 3, Table 2). These results are similary that previous studies have reported that melon fruits differ in size and shape and β-carotene content affects the skin color and fruit appearance (Chikh et al., 2010; Monforte et al., 2004; Olivas and Barbosa-Canovas, 2005; Liu et al., 2020). As shown in Table 2, the length of seed for Group Ⅰ was 1.47 ㎝, longest. β-carotene content of Group Ⅰ melons was 62.98 ㎎/㎏. Resources with long seed-length and high β-carotene content were S/no. 2 (IT K153005), S/no. 38 (IT K190933) originated from FRA and ALB, respectively. Group Ⅲ had the longest length of leaf 22.21 ㎝ and a β-carotene content of 14.91 ㎎/㎏. Resources with long leaf-length and high β- carotene content were S/No. 30 (IT 190392) and S/No. 28 (IT K190326) originated from UZB. Group Ⅱ and Ⅴ had short length of joints of 9.28 ㎝ and 9.3 ㎝ with high β-carotene contents of 60.13 ㎎/㎏ and 38.8 ㎎/㎏, respectively. Resources with short length of joint and high β-carotene content were S/No. 40 (IT K260962), S/No. 56 (IT 297270) originated from USA.
Table 2.
Grouped the morphological characters, β-carotene content and cucumisin activity of 58 melon fruits
| Group | LLz (㎝) | LJy (㎝) | WFx (㎝) | LSv (㎝) | WSu (㎝) | β-carotene content (㎎/㎏) | cucumisin (U/㎎) | ||||
| UV | MR | HPLC | UV | MR | |||||||
| Ⅰ |
21.55± 6.90 |
11.77± 6.56 |
14.59± 1.69 |
1.47± 4.39 |
0.49± 0.04 |
82.34± 43.34 |
77.71± 38.02 |
62.98± 29.81 |
1.23± 0.53 |
1.21± 0.50 | |
| Ⅱ |
13.86± 15.49 |
9.28± 6.22 |
9.14± 7.15 |
0.81± 0.03 |
0.39± 0.01 |
86.75± 64.99 |
82.88± 50.85 |
60.13± 36.13 |
1.8± 5.17 |
1.73± 4.94 | |
| Ⅲ |
22.21± 1.30 |
11.65± 2.12 |
10.39± 5.49 |
1.05± 0.02 |
0.46± 0 |
25.56± 2.09 |
24.5± 2.34 |
14.91± 1.98 |
1.72± 1.02 |
1.67± 0.86 | |
| Ⅳ |
15.52± 2.86 |
11.59± 13.36 |
9.44± 2.50 |
0.91± 0.07 |
0.4± 0.01 |
86.25± 61.70 |
82.95± 57.64 |
57.31± 39.77 |
1.16± 0.92 |
1.12± 0.81 | |
| Ⅴ |
17.15± 2.65 |
9.3± 8.00 |
45.9± 1.31 |
0.8± 0.08 |
0.35± 0.01 |
54.65± 19.91 |
49.65± 18.91 |
38.8± 12.30 |
1.35± 1.81 |
1.3± 1.62 | |
Based on studies that melon flesh contains carotenoids such as α-carotene, β-carotene, and lutein that can reduce the risk of cancer and heart disease (Ahmad et al., 2019; Chikh-Rouhou et al., 2019; Grazielle et al., 2021), S/No. 2 (IT K153005), S/No. 38 (IT K190933), S/No. 30 (IT 190392), S/No. 28 (IT K190326), S/No. 40 (IT K260962), S/No. 56(IT 297270) can be used as basic data for research on highly functional melon varieties. Combining the results of previous studies, which reported that melon fruits differ in size and shape and that β-carotene content influences the color of skin, and the shape of fruit (Sies and Stahl, 1995; Wackerbarth et al., 2009), and our studies that screened the significance of β-carotene and cucumisin content, we could predict β-carotene content indicators according to the color of fruit, the length of leaf, the width of fruit, the length of joint, and the length of seed. Collectively, these results the morphological properties of melons could help predict indicators of β-carotene content and help develop functional sarcoma and farmhouse cultivation.
Analysis of β-carotene and cucumisin using spectrophotometric and HPLC methods Method validation
The extraction of β-carotene was conducted using a mixture of solvents and reagents according the previously reported method, with some modification. In our experiment, distilled water was added at the first stage of the extraction step as opposed to the procedure followed by Davis et al. (2008-2009). Melon fruit flesh sample contains a high level of sugar which is less soluble in organic solvents and form aggregates that make dissolution slow. Preliminary experiments showed that the β- carotene content highly varied with the degree of dissolution of the sample with an organic solvent added at the first step. The addition of water at the primary stage allowed a better dissolution of the extraction mixture.
Calibration curves were prepared from an average of three independent β-carotene standard solutions of serial dilutions. According to the calibration experiment, the UV, MR, and HPLC methods showed linearity in the range between 0.19 and 6.25, 0.19 and 12.50, and 0.19 and 25.00 ㎍/mL of β- carotene concentrations, respectively. Recovery test, inter- and intra-day precision, limit of detection (LOD) and limit of quantification (LOQ) of β-carotene analysis were performed using two melon fruit samples. The recovery test verified the efficiency of the methods for extraction and analysis of β- carotene. The data obtained by spiking 12.5 to 100 ㎎ standard of β-carotene showed a mean recovery of 88.47 to 100.80%, 87.17 to 103.30%, and 88.71 to 101.11% using UV, MR, and HPLC methods, respectively, suggesting the reliability and accuracy of the methods. Recovery test results are tabulated in Table 3.
Table 3.
Recovery test results of HPLC and spectrophotometric methods using two melon fruit samples
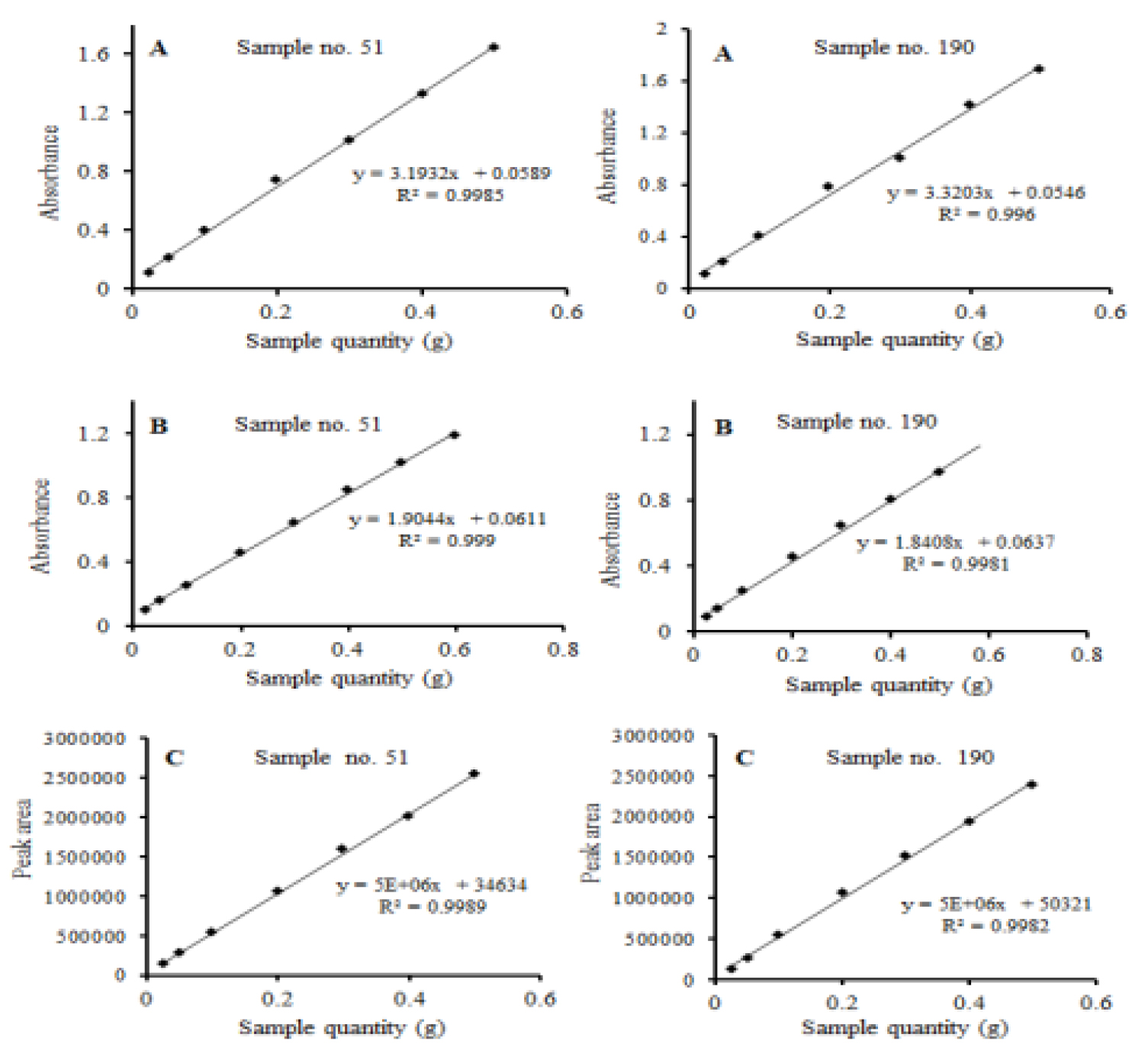
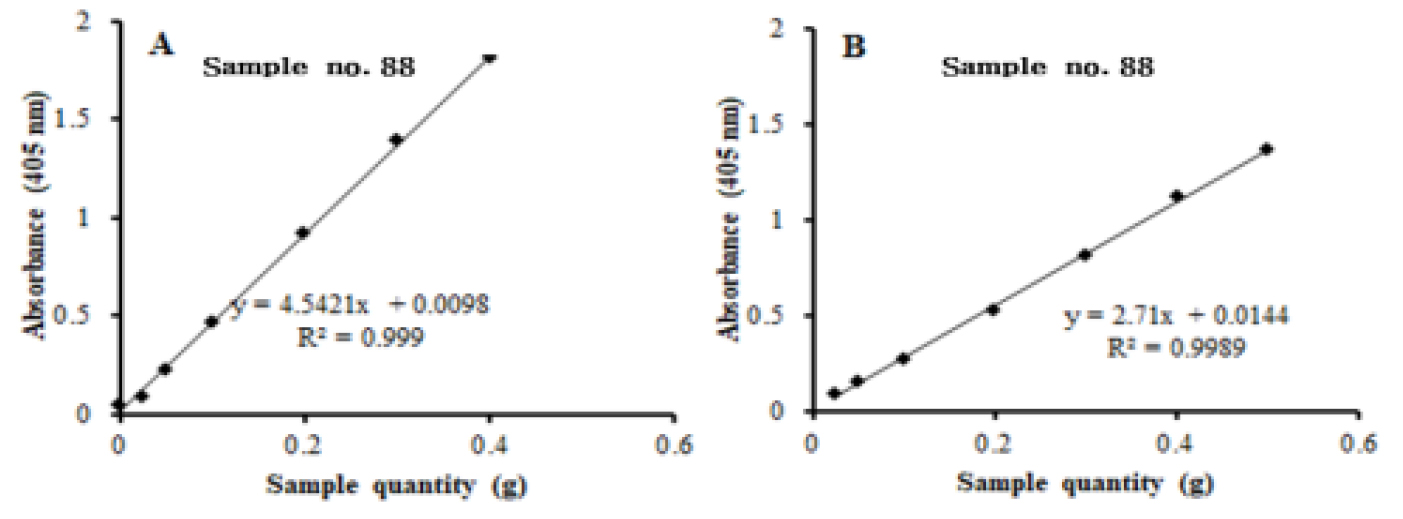
The precision of the method was determined as the percentage of the ratio of the standard deviation to the mean value (relative standard deviation, RSD) of inter-day (n=5) and intra-day (n = 5) analysis. The inter-and intra-day precision results are summarized in Table 4, Table 5. The limit of detection (LOD, 3.3*σ/S; σ indicates the standard deviation of Y- intercept while S stands for the slope of the calibration curve) and the limit of quantification (LOQ, 10*σ/S) were 0.0266, 0.0236, 0.0491 ㎍/mL and 0.0798, 0.0708, and 0.1474 ㎍/mL, for UV, MR, and HPLC methods, respectively, demonstrating each of the methods were efficiently sensitive enough to detect the amount of β-carotene in melon samples. The effect of sample size (sample to solvent ratio) on the response of the instruments for was evaluated in two melon fruit samples for HPLC and spectrophotometric methods β-carotene (Fig. 4) and cucumisin (Fig. 5). A linear response was exhibited in the range of 0.2 to 0.4 g dry weight of melon fruit samples in both instruments. β-carotene and cucumisin in melon pulp are known to be effective in anticancer and anti-cerebrovascular diseases, and have recently attracted attention as functional fruits (Priscilla et al., 2020).
Table 4.
Inter- and intra-day precision results of HPLC and spectrophotometric methods using two representative melon fruit samples for β-carotene analysis
Table 5.
Inter- and intra-day precision results of spectrophotometric methods using two representative melon fruit samples for cucumisin analysis
| Method | Sample | Intra-day precision (n = 5) | Inter-day precision (n = 5) | |||
| Activity (U/㎎, dw) | RSD (%) | Activity (U/㎎, dw) | RSD (%) | |||
| UV | Sample C | 1.86 | 0.54 | 1.87 | 0.57 | |
| MR | Sample C | 1.77 | 2.02 | 1.76 | 0.82 | |
In this study, β-carotene, cucumisin content, and morphological properties of 58 melon resources were evaluated. It was measured based on the International Union for the Protection of New Varieties of Plants (UPOV, 2019), and the shape of fruit (SF), the color of flesh (CF), width of fruit (WF), strength of fruit flesh (SFF), color of fruit skin (CFS), the length of seed (LS) and width of seed (WS). β-carotene in melon resources contained an average of 48.76 ㎎/㎏, and the content range was 0.37-188.95 ㎎/㎏. The β-carotene high- content resource was S/No. 44 (188.95 ㎎/㎏), which was about 1.5 times higher than that of the control group. As a result of analyzed the β-carotene and cucumisin content and morphological properties of melon, it was determined into five groups. These results are similar to reports that melon fruits differ in size and shape and that β-carotene content affects the color of fruit and the shape of fruit (Li et al., 2019). Resources with high seed length and β-carotene content were S/no. 2 (IT K153005) and S/no. 38 (IT K19093). Resources with long leaf length and β-carotene content were S/No. 30 (IT 190392) and S/No. 28 (IT K190326). The resources with a short length and high carotene content were S/No. 40 (IT K26092) and S/No. 56 (IT 297270). These resources could predict indicators of β-carotene content as morphological properties of melons, and help develop functional sarcoma and farmhouse cultivation.