Introduction
Materials and Methods
Results and Discussion
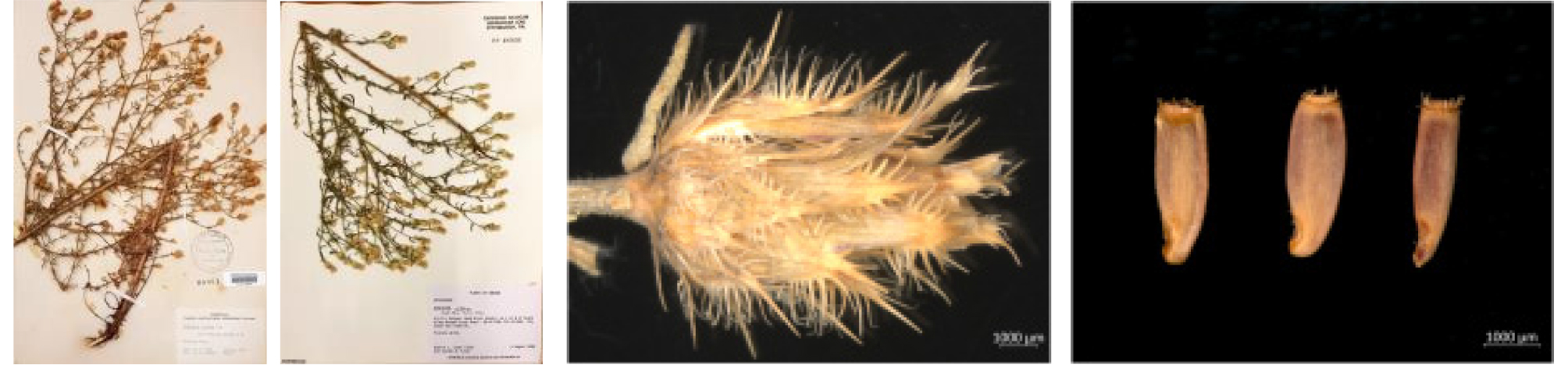
Centaurea maculosa Lam., Encycl. [J. Lamarck & al.] 1(2):669 (1785)
Centaurea diffusa Lam., Encycl. [J. Lamarck & al.] 1(2): 675 (1785)
Mikania micrantha Kunth in Humboldt et al., Nov. Gen. Sp. 4, ed. f°:105 (1818)
Cenchrus echinatus Linnaeus, Sp. Pl. 2: 1050 (1753)
Neyraudia reynaudiana (Kunth) Keng ex Hitchcock, Amer. J. Bot. 21: 131 (1934)
Brachiaria mutica (Forsskål) Stapf in Prain, Fl. Trop. Africa. 9: 526 (1919)
Vulpia bromoides (L.) Gray Nat. Arr. Brit. Pl. ii. 124 (1821)
Lolium persicum Boissier & Hohennacker in Boissier, Diagn. Pl. Orient., ser. 1. 13: 66 (1854)
Setaria palmifolia (J. Konig) Stapf, J. Linn. Soc., Bot. 42: 186 (1914)
Prosopis glandulosa Torr. in Ann. Lyc. N.Y. 2:192 (1827)
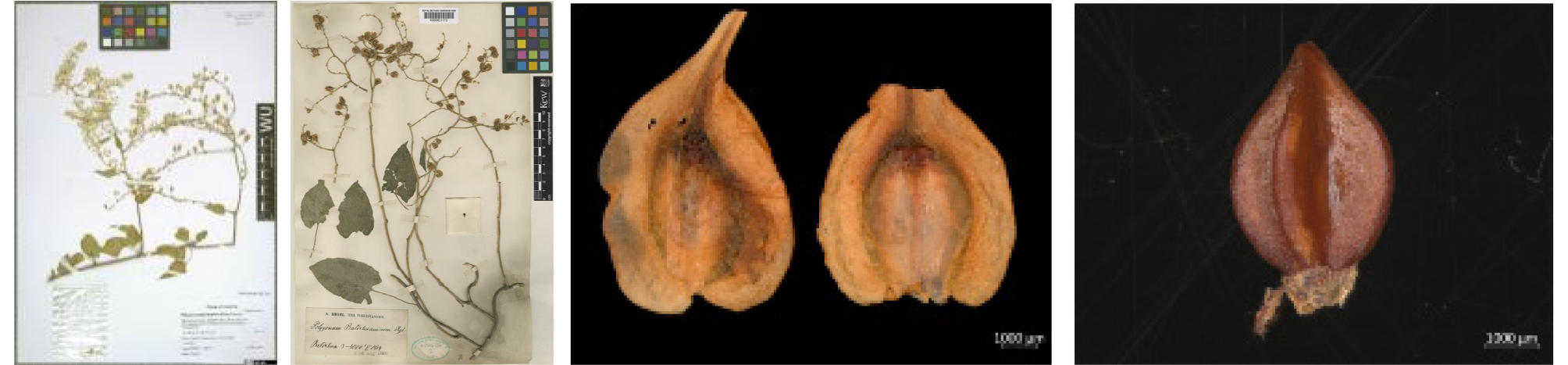
Fallopia baldschuanica (Regel) Hobub. in Folia Geobot. Phytotax. 6:176 (1971)
Introduction
The characteristics of species are the result of evolutionary adaptations occurring over many thousands of generations. There are more than 350,000 species of plants worldwide, and also nearly 4,000 indigenous plants to Korea are well adapted to various ecological environments. The importance of biodiversity is well recognized and largely considered an essential factor for human activities as a facilitator of sustainable development. Since the start of the 20th century, the Invasive Alien Species (IAS) have been a key factor affecting biodiversity and ecosystem services (IUCN, 2010). They are possible threats to biodiversity and ecosystem stability and widely held to be responsible for the decline of native species richness and the local extinction of certain species. In addition to their deleterious impact on biodiversity, the IAS also negatively impact human communities and economies (Gaertner et al., 2009; Hulme, 2009; IUCN, 2014; Pimentel et al., 2005).
Invasive species such as plants, animals, disease agents and other organisms taken beyond their natural range by people, deliberately or unintentionally become destructive to the environment or human livelihoods. The term ‘Invasive alien plants’ that we use in this study is defined as taxa including seeds, roots, rhizomes and stolons listed by the Korean government as the possible causes of serious environmental risk in Korea.
There is a rapidly increasing rate of invasive plants in Korea [for example, 110 species of invasive plants in 1980 (Yim and Jeon, 1980), 181 species in 1995 (Park, 1995), 292 species in 2003, 303 species in 2007, 321 species in 2011 (Korea National Arboretum, 2012) and 334 species in 2019 (National Institute of Ecology, 2019)]. In Korea, various acts and plans put in place by the Ministry of Environment have been designed to protect biodiversity by 1) enhencing invasive alien species control system, 2) expanding conservation regions, and 3) making lists of national biological resources including endangered species (Ministry of Environment, 2015b; Ministry of Environment, 2017; National Institute of Ecology, 2019). These invasive plants have been found all over the South Korea, from Jeju Island to DMZ (Kim et al., 2006; NamGung et al., 2019).
Based on the patterns, characteristics, and status of ecological and agricultural risks that were caused by introduced harmful plants, it is necessary to conduct a technical field survey of putative invasive alien plants and to find out their characteristics and strategy to remove them immediately if introduced. The most important approach is to prevent the inflow of potentially invasive alien species on the border through a strict quarantine process up to now. On the circumstance of global climate change, annual average temperature in Korea has been rising during a hundred years as +0.18℃/10 year (National Institute of Meteorological Sciences, 2018). As a temperate climate region country, annual average temperature of Korean peninsula was 14.1℃ in recent 10 years (2011~2017; National Institute of Meteorological Sciences, 2018), and annual precipitation was 1,332 ㎜ (1971~2013) including heavy monsoon in summer (Ministry of Environment, 2015). This study considered climate similarity with several temperate region states (Texas, Georgia, South and North Carolina, Virginia, etc.) in USA which numerous alien plants have been invaded.
Then, the main goal of this study is to provide useful basic information of putative invasive alien species in Korea which have been designated as harmful species by the Ministry of Environment in 2016 to prepare the management strategy for prevention. Also we present a detailed taxonomic information including descriptions and keys, agricultural and environmental risk assess analysis of 11 putative invasive alien plant species.
Materials and Methods
To figure out of the putative invasive alien plants of Korea, we investigated the literature, specimens, and other information to suggest a comprehensive reference including seeds morphology. We conducted the field survey of putative invasive alien plants in the southeastern region of the United States from 8th to 17th of July, 2018 including Texas, Florida, Georgia, South Carolina, North Carolina, Virginia, and Maryland. Through the habitat survey to inquiry the distribution status, ecological characteristics, and weed-risk assessments. Eleven of the 41 putative invasive species surveyed are presented along with descriptions of their natural habitat and taxonomic key with diagnostic characters : Centaurea maculosa Lam., Centaurea diffusa Lam., Mikania micrantha Kunth. (Asteraceae), Cenchrus echinatus L., Neyraudia reynaudiana (Kunth) Keng ex Hitchcock, Brachiaria mutica (Forsskål) Stapf, Vulpia bromoides (L.) Gray, Lolium persicum Boissier & Hohennacker, Setaria palmifolia (J. Konig) Stapf (Poaceae), Prosopis glandulosa Torr. (Fabaceae), Fallopia baldschuanica (Regel) Hobub. (Polygonaceae) (Table 1, Fig. 1).
Table 1.
Investigated plants list in this study
We prepared the photograph of investigated species and habitat. Also we observed the the dried voucher specimens from several herbaria in US including UT (Austin), UNC (Chapel-Hill), USNH (Washington DC), UF (Gainsville) and etc.
Results and Discussion
Centaurea maculosa Lam., Encycl. [J. Lamarck & al.] 1(2):669 (1785)
Korean Name : Bun-hong-su-re-guk-hwa (분홍수레국화; Ministry of Environment and National Institute of Ecology, 2014)
Common Name : Spotted knapweed.
Taxonomic description and distribution characteristics
Taxonomic description Biennial or short-lived perennial; a taproot stout, exude chemicals such as catechin, inhibit growth of other plants. Stem erect, 60~120 ㎝ tall at anthesis, pubescent, slender branches 1~20, especially in upper part. Leaves alternate, pale green; 3~9 ㎝ long, in leaves on the lower part of the stem, margin dentate, irregular or cleft to middle parts of the midvein, adaxial surface coarse; leaves on the upper part of the stem having flowers almost linear; in the first year, radical leaves form a rosette, gray green color; in the next year stems emerge. Flowers in June to August, thistle- like, pink to purple. Capitula at end of stem and branches 2 ㎝ in diam. Involucre densely numerous, black spotted because of phyllaries apex; phyllaries striate longitudinally, apex acute, black, margin pectinate. Fruits in July to September, Achene brownish, below 6.5 ㎜ long, one side of lower part caved in, upper part (apex) bristle-like short pappus. Chromosome number 2n=36.
Growing conditions This species grows on stream banks, pastures, roadsides and many open, disturbed areas.
Origin eastern Europe and Asia Minor.
Invaded areas It has been introduced to North America (US and Canada, etc.) in the late 1800’s
Current state of designations In the late 1800’s, this species likely spread to North America in an alfalfa shipment. In the year 2000, it was reported in 45~50 states in the US. National Invasive Species Information Center (The United States National Agricultural Library /NAL) put on the invasive species lists. By the Montana Field Guide, this species is on the list, Noxious Weed/ Priority 2B.
Specimens observed
[U. of Florida Herbarium] 57449 (R. L. McGregor, 1940. 7. 13. United States) ; 113483 (D. R. Windler, 1971. 8. 8. United States) ; 58858 (S. C. Hood, 1949. 7. 8. United States) [Carnegie Museum Herbarium] 503561 (Joseph A., 2003. 8. 7. United States) ; 502238 (Joseph A., 2002. 8. 10. United States) ; 500525 (Joseph A., 2002. 8. 8. United States) [United States National Herbarium] 3179779 (Richard R. Halee, 1987. 8. 14. United States)
Key compared with Korean similar species
This species is similar in morphology to Centaurea cyanus L. introduced in Korea. Key will be written in following Centaurea diffusa Lam. together.
Harmfulness of weeds
This plant which is originated in eastern Europe and Asia minor shows very high spreading after invasion on the farms and ranches (Fig. 2). We investigated in some fields and road side of Texas. And this plants are biennial or perennial herbs which form the broad underground root system. Also approximately 1,000 fertile seeds per individual are produced in wild habitat, and it is possible to produce more seeds in high humid condition than in a dry weather. This weeds are distributed broadly in eastern and western parts except midwest dry area in United States. We found this plants at the foot path of natural parks in Florida and Virginia. We wonder the risk of this plant not by the toxicity but by the productivity reducing effect of vegetation sources by massive high spreading. Now this plants are expanding the invasive area between 10℃ to 32℃ temperature in North America. If this plants would be introduced in Korean peninsula, it is possible to spread out all the area rapidly.
Weed risk assessment
By the result of weed risk assessment criteria (Animal and Plant Quarantine Agency in Korea), degree of risk is 68.9% (Table 2). We suggest that this species should to be registered to control in law.
Centaurea diffusa Lam., Encycl. [J. Lamarck & al.] 1(2): 675 (1785)
Korean Name : Pau-jin-su-re-guk-hwa (퍼진수레국화; National Institute of Ecology, 2019)
Common Name : Diffuse knapweed, White knapweed, Tumble knapweed.
Taxonomic description and distribution characteristics
Taxonomic description Annual or biennial Herb. In the first year, radical leaves form a rosette after germinating, in the next year stems emerge and flowers bloom. Stems erect or diffuse, branched from base; stem and branches densely strigose and sparsely arachnoid; 10~60 ㎝ tall. Leaf blade bipinnatisect, adaxially scabrous; radical leaves and lower stem leaves petiolate, middle and upper stem leaves sessile, linear, margin entire. Capitula numerous, paniculate, one or sometimes 2~3 at end of stem and branches, capitulum urn- shape, 1.5~2 ㎝ long. Phyllaries in 5~6 rows, yellowish, brownish at margin; outer and middle phyllaries lanceolate to elliptic, 3~7 ㎜ long, 1.5 ㎜ wide, appendage straw-colored, 1~5 pectinate spines on each side ending in a 2~4 ㎜ arcuate-patent spine; inner phyllaries linear, 8 ㎜ long, 1 ㎜ wide, appendage scarious. Sterile florets few, as long as bisexual florets, not radiant; bisexual florets usually 12; flowers in July to September, corolla white or occasionally pink. Achene dark brown or black with paler stripes, 2~3 ㎜ long, narrowly ovoid, sparsely pilose; apical rim inconspicuous at apex, less than 1 ㎜; pappus absent; fruits in July to September, ca. 18,000 fruits in a plant. Chromosome number 2n=18, 36 (Watson and Renney, 1974).
Growing conditions This species prefers arid or semi-arid habitats. Infestations stop abruptly with an increase in soil moisture near temporary and permanent streams or with flooding and irrigation (Berube and Myers, 1982). Other areas of invasion include road and railway, waterways, gravel pits and industrial areas (Roche and Roche, 1988).
Origin SW Asia (Asia Minor; Turkey, Syria), Europe (Balkan peninsula, Bulgaria, Greece, Rome, Ukraine, S Russia).
Invaded areas In 1907, this species was found in an alfalfa field in Washington. Now present in at least 19 states in the US and western Canada and China (Liaoning).
Current state of designations It is a restricted noxious weed in 13 US states and 4 Canadian provinces.
Specimens observed
[U. of Florida Herbarium] 60851 (S. C. Hood, 1950. 7. 24. United States) [Carnegie Museum Herbarium] 497633 (Bonnie L. Issac, 2000. 8. 5. United States) ; 250687 (B. Kuzmanov, 1972. 6. 26. Bulgaria) ; 300150 (Baschens, 1934. 9. 16. United States) [United States National Herbarium] 02161054 (E. Panou, 1934. 8. 13. Poland)
Key compared with Korean congeneric species (Korean name)
1. Apex of main phyllaries not spiny, pappus present............................................................................................................2
2. Annual, pappus stiff bristle, 2~4 ㎜ long................................................................................Centaurea cyanus(수레국화)
2. Biennial, pappus stiff bristle, ca. 1 ㎜ long..................................................................Centaurea maculosa(분홍수레국화)
1. Apex of main phyllaries spiny, pappus absent..............................................................Centaurea diffusa (퍼진수레국화)
Harmfulness and characterisitics of weeds
This is annual or perennial honey plant which originally distributed in western Asia and Europe. Very small seeds (2-3 ㎜ in size) would be released unintendedly by water, wind, cattle and automobiles (Fig. 3). This plant grows well in dry land area and is easy to spread out rapidly to the disturbed areas such as road sides, rail way sides, meadows and forest edges. It is not easy to be infiltrated in normal farm area because of humid weakness, but it is highly possible to spread out in of well-drained paddy field including sands and pebbles. Spines of plants hurt the digestive organs of cattle, and chemicals from the roots react the allelopathy to prevent the growth of other plants. Now this plants are adapted broadly in temperate regions and growing well in dry and half-dry area between 7.2℃ to 9.4℃ in annual temperature and 240 ㎜ to 420 ㎜ in annual precipitation. If this species would be introduced in Korean peninsula, it is highly possible to spread out all the area rapidly.
Weed risk assessment
By the result of weed risk assessment criteria (Animal and Plant Quarantine Agency in Korea), degree of risk is 60.6% (Table 2). We suggest that this species should to be registered to control in law.
Mikania micrantha Kunth in Humboldt et al., Nov. Gen. Sp. 4, ed. f°:105 (1818)
Korean Name : Deong-gul-deung-gol-na-mul (덩굴등골나물; Ministry of Environment and National Institute of Ecology, 2014)
Common Name : Mile-a-minute vine, American rope, Bittervine, Chinese creeper, Climbing hemp vine, Mikania vine.
Taxonomic description and distribution characteristics
Taxonomic description Vines, vigorously growing perennial. Stem usually terete, slender, branched, yellowish or brownish, slightly striate, glabrate to sparsely puberulent, up to 6m. Leaves opposite, petiole 1~6 ㎝, leaf blade ovate, 4~13 ㎝ long, 2~9 ㎝ wide, veins 3~7, both surfaces glabrous, with numerous glandular spots; base cordate or deeply cordate, margin entire or coarsely dentate, apex shortly acuminate. Compound inflorescence corymbose panicle; capitula clustered on branches; phyllaries 4, oblong, apex shortly acuminate, ca. 3.5 ㎝ long, glabrous or puberulent. Corolla white, 2.5~4 ㎜ long, tube narrow, limb broadly campanulate, inside papillate. Achene 1.5~2 ㎜ long, dark grey to black, somewhat flattened, 4-ribbed, with short and white hairs along the rib, with many scattered glands; pappus white, below 3 ㎜ long (usually 1.2~1.8 ㎜ long). Achenes are dispersed by wind. Flowers and fruits year-round, especially flowers in September to October, fruits in November to December, ca. 20,000~40,000 achenes in a plant. Chromosome number 2n=36, 72.
Growing conditions It is usually found in damp, lowland clearings or open areas, where there is adequate temperate, light and rainfall. It also grows along streams and roadsides, along edges of forests and forest plantations, along fence- lines, in pastures and wastelands and on and among tree crops (Parham, 1958; Parham, 1962; Adams et al., 1972; Waterhouse and Norris, 1987; Holm et al., 1991; Day et al., 2012).
Origin South and Central America.
Invaded areas This species was intentionally introduced into the Asia-Pacific region (Taiwan for soil conservation; India, Indonesia and Malaysia for ground cover) (Ismail, 2001), but now widely spread in South Pacific (Fiji, Samoa), Southeast Asia (Thailand, India, Sri Lanka), China, Nepal, Guam and Australia.
Current state of designations Control of this species is difficult as it produces are large number of seed, can readily shoot from runners and suckers and can regenerate from stem fragments. This species has been the target of a biological programme in many countries. It was recently listed as a priority environmental weed in at least one Natural Resource Management region and appears on the Northern Australia Quarantine Strategy (NAQS) list. Because of its invasiveness in other parts of the world, this species is also listed in the Global Invasive Species Database, and is regarded to be in the top 100 of the world's worst invasive alien species.
Specimens observed
[Carnegie Museum Herbarium] 295839 (Sue A. Thomson, 1983. 7. 14. Ucuador) ; 352161 (Ricardo Callejas, 1978. 10. Colombia) ; 343581 (A. Gentry, 1980. 4. 1. Peru) ; 510682 (Sue A. Thomson, 1991. 10. 10. Dominican Republic) ; 400544 (J. S. De La Cruz, 1923. 9. 18. British Guiana) [United States National Herbarium] 3291563 (Grady L. Webster, 1993. 9. 1. Ecuador) ; 3176386 (Vlastimil Zak., 1988. 4. 11. Ecuador)
Key compared with morphologically similar species of related Korean genus (Korean name)
1. Vine, leaf base cordate or deeply cordate, florets in a capitulum 4...........................Mikania micrantha(덩굴등골나물)
1. Erect or spreading, leaf base round, obtuse or truncate, florets in a capitulum 10~30...................................................
...............................................................................................................................................Ageratina altissima (서양등골나물)
Characterisitics of weeds
This perennial species is originated from central and southern America which are used for medicinal and edible, and occasionally it is planted to prevent the soil erosion of rubber farm surface. A single mikania plant had been reported to produce up to 54,000 seeds annually (Fig. 4). The seeds by the pappus can be scattered by the wind or mixed into other crops to spread. This climbing plant spreads out rapidly and afflict other plants to be defoliated by blocking the light over the crops, shrubs, trees, and even wall or fence. Based on the broad climate condition of natural habitat (relative warm temperate to subtropical) with annual average temperature between 13℃ to 30℃ with annual average precipitation between 1,000㎜ to 3,000㎜. If this plants would be introduced in Korea, it is possible to spread out in southern parts of peninsula and Jeju islands.
Weed risk assessment
By the result of weed risk assessment criteria (Animal and Plant Quarantine Agency in Korea), degree of risk is 55.7% (Table 2). We suggest that this species needs to be registered to control temporarily in law and to investigate the potential risk.
Cenchrus echinatus Linnaeus, Sp. Pl. 2: 1050 (1753)
Korean Name : Mi-guk-ga-si-pul (미국가시풀; Ministry of Environment and National Institute of Ecology, 2014)
Common Name : Southern sandbur, Bur grass, Burgrass, Burr grass, Common sandbur, Golden grass, Hedgehog grass, Mossman river grass, Sand bur, Sand burr.
Taxonomic description and distribution characteristics
Taxonomic description Annual, erect, forming loose tufts. Culms (stems) 15~90 ㎝ high, lower parts of the culm sometimes geniculate, usually rooting at basal nodes, usually flattend, dark green. Leaf sheaths keeled, usually imbricate at base, with hairs at the mouth of sheath; leaf blades linear or linear-lanceolate, 5~25 ㎝ long, 3~12 ㎜ wide, glabrous or pubescent; ligule 0.5~2.0 ㎜ long, ciliate, membranous; auricles absent. Inflorescence densely cylindrical spike, 3~10 ㎝ long, 1~2 ㎝ wide, burrs contiguous, rachis scabrous, strongly undulate; burrs globose, 0.4~10 ㎜ long, upper part truncate, stipe pubescent, all spines and bristles retrorsely barbed, spine 2~5 ㎜ long; inner spines connate for 1/3~1/2 their length, the flattened tips triangular, erect or bent inward; outer spines in 2 divergent whorls, a median whorl of rigid spines equalling the inner teeth, an outer whorl short, few, bristles slender, tips of the spines purple with increasing maturity. Spikelets 2~4 in each burr, 4.5~7.0 ㎜ long, 1~2 ㎜ wide, narrowly ovate; lower florets usually sterile or occasionally male, upper florets bisexual, stamens of male floret 3, pistil of bisexual florets 2 feathery stigmas. lower glume 1/2 spikelet length; upper glume 2/3~3/4 spikelet length. Caryopses broadly oval or ovoid, 1.5~3.2 ㎜ long, 1.3~2.2 ㎜ wide, brownish, tip flattened. Flowers and fruits in summer to autumn. Chromosome number 2n=34, 68.
Growing conditions This species is a weed of tropical, sub-tropical, warmer temperate, semi-arid and arid climates. This species grows well in footpaths, urban parks, lawns, roadsides, disturbed sites, waste areas, grasslands, open woodlands, wetlands, pastures and crops. It is an aggressive competitor that colonizes sandy soils, particularly along the coast, and can have a significant impact on coastal sand dune communities.
Origin Central America and tropical North America.
Invaded areas This species is now widespread throughout the tropical regions of the world (including China, Taiwan and Australia) and naturalized on that regions.
Current state of designations This species is mainly regarded as an environmental weed in northern Queensland, the Northern Territory, and the northern parts of Western Australia. This species was also recently listed as a priority environmental weed in at least one Natural Resource Management region. Also this is listed among the top ten environmental weeds in the Kimberley and Pilbara coastal regions of Western Australia. It is also regarded as an environmental weed in the New South Wales north coast region and in south-eastern Queensland, where it appears on the list of the top 200 invasive plants.
Specimens observed
[Carnegie Museum Herbarium] 316967 (M. G. Silva, 1977. 6. 25. Brazil) ; 359774 (Renvoize, 1980. 12. 5. Ecuador) ; 327643 (Carlos Mejia, 1981. 9. 12. Honduras) ; 222731 (Gerrit Davidse, 1910. 5. 9. Pines Island) [United States National Herbarium] 3193464 (J. R. Grant, 1991. 5. 26. Costa Rica) ; 572313 (Edward Palmer, 1907. 4. 9. Mexico)
Key compared with Korean congeneric species (Korean name)
1. Burr is ovoid or ellipsoidal, flattened and concate spines consist of several whorls.........................................................
...............................................................................................................................................Cenchrus longispinus (대청가시풀)
1. Burr is globoid, base of flattened spines on burr consist of a whorl............................Cenchrus echinatus(미국가시풀)
Characterisitics of weeds
This species is an annual herb which originally distributed in central and northern tropical America. Because of the strong invasive nature of arable land, it is treated and managed as a weed in many agricultural areas worldwide. Spiny seeds can be spread easily by attaching to the clothes or animal hairs (Fig. 5). Spines can cause skin entrapment or scarring, and when mixed with hay, they lower the digestion rate and appetite of cattle. In intrusion areas, they are mainly distributed in roadside, disturbance areas, coastal sand dunes and wetlands, and the rate of spreading is faster in areas where humidity is high than in dry conditions. We investigated the distribution in humid disturbed areas in Texas, United States. There are no cases of cultivation or commercial trade of this plant, and many countries have designated it as a quarantine weed to carry out many efforts to prevent it. It is effective to remove all the plants including the roots by eradication control before the fruiting. In North America, plants are spreaded to the areas with an average annual temperature of 24℃ to 28℃. Cenchrus longispinus, other invasive species in the same genus, has already been invaded and is spread throughout the islands in West Sea, Korea. Then if this species is introduced in Korean peninsula, it could spread along the coast of Jeju Island and the West Sea.
Weed risk assessment
By the result of weed risk assessment criteria (Animal and Plant Quarantine Agency in Korea), degree of risk is 65.6% (Table 2). We suggest that this species should to be registered to control in law.
Neyraudia reynaudiana (Kunth) Keng ex Hitchcock, Amer. J. Bot. 21: 131 (1934)
Korean Name : Beo-ma-gal-dae (버마갈대; Ministry of Environment and National Institute of Ecology, 2014)
Common Name : Burma reed, Cane grass, Silk reed, False reed.
Taxonomic description and distribution characteristics
Taxonomic description Perennial, caespitose from a short woody rhizome; Culms robust, erect, 1~3m tall, 3~10 ㎜ in diameter, usually branched fasciculately, internodes somewhat glaucous, node purple. Leaf sheaths glabrous, mouth of sheath pilose; leaf blades flat or involute, 20~70 ㎝ long, 0.4~1 ㎝ wide, glabrous or adaxial surface pilose, apex acuminate; ligule 1~2 ㎜ long. Inflorescence panicle, wide spread, spikelets loose or dense, 30~70 ㎝ long, branches slender and nodding; pedicels 1~2 ㎜ long. Spikelets 6~9 ㎜ long, florets 4~10, lowest floret sterile, resembling glumes or somewhat longer than glume, glumes golden-brownish or puplish, glabrous, subequal, 2~3 ㎜ long, acut; lemmas purplish, ca. 4 ㎜ long, ciliate on later veins, hairs on ciliate ca. 2 ㎜ long, awn recurved, 1~2 ㎜ long. Flowers and fruits in August to December (April to October in south Florida, US). Seeds 1.5~3 ㎜ long, narrowly elliptic. This species resembles several other tall grasses, including Phragmites communis, Arundo donax. Chromosome number 2n=40.
Growing conditions This species grows well in streamside, hill slopes, rocky places, old walls, up to alt. 2,000 m.
Origin This species is widely distributed in warm, subtropical habitats in Southeast Asia and Indomalaya realm, including portions of Japan, southern China, Vietnam, Laos, Cambodia, Thailand, Malaya, Myanmar (Burma), Bhutan, Nepal, and eastern India.
Invaded areas This species was naturalized in Australia, islands on the Pacific, USA, ect.
Current state of designations This species was listed as “Florida Noxious Weed List”. Risk assessments of this species have been conducted for the USA, Australia (Koop et al., 2011) and the Pacific Islands (PIER, 2014). All found the species to be a high risk. In South Africa this species has been listed as a prohibited species (Molewa, 2004).
Specimens observed
[U. of Texas Herbarium] 00208989 (Walter Koelz, 1948. 10. 24. India) ; 00208988 (Walter Koelz, 1948. 9. 16. India) ; 00208990 (Steve L. Orzell 19903. 6. 13. United States) [United States National Herbarium] 1507941 (Gerald F. Guala, 1922. 10. French Indochina) ; 1507941 (Park Shik Ling, 1935. China) ; 2683224 (Shiu Ying Hu, 1968. 12. 8. Hong Kong) [New York Botanical Garden] 1758485 (M. Nee, 1990. 12. 23. United States) ; 1758489 (D. S. Correll, 1977. 12. 27. United States) ; 1758488 (Harold N. Moldenke, 1930. 1. 17. United States)
Key compared with morphologically similar species of related Korean genera (Korean name)
1. Long hairs of floret is attached on callus...............................................................................................................................2
2. Lower glume is ca. 1/2 of lower lemma, stolons creep straight, node glabrous...................Phragmites australis(갈대)
2. Lower glume is ca. 1/2~3/5 of lower lemma, stolons creep zig-zag, node pubescent..Phragmites japonica(달뿌리풀)
1. Long hairs of floret is attached on lemma............................................................................................................................3
3. Long hairs is attached on basal part of lemma abaxially, awns from the cleft apex erect..........Arundo donax (물대)
3. Long hairs is attached on lateral vein of lemma, awns from the midvein extension recurved.......................................
...............................................................................................................................................Neyraudia reynaudiana(버마갈대)
Characterisitics of weeds
This perennial herb originated from temperate and subtropical regions of Southeast Asia and grows well, even in high-altitude areas. Neyraudia reynaudiana: i) is often used for ornamental purposes and can help retain soil structure, ii) produces large quantities of seeds that are dispersed by wind and can attach to animals for propagation, iii) also spreads by fragmented rhizomes, which may be transported by construction machinery. The inflowing process is firstly intruded into roads, open lands, forest edges, etc. and the plants easily spread to adjacent areas when colonies are formed (Fig. 6). This plant is resilient and tolerates a variety of soil, light and moisture conditions, however, seems to thrive most on infertile, rocky, dry locations in full sun. As the height of this plant reaches up to 3 meters, it can inhibit the growth of lower-class plants due to shade formation, and may reduce the production of crops in agricultural areas. Neyraudia reynaudiana is especially vulnerable to fire because of its dense growth characteristics, however, it can quickly recur after the fire with rapid growth and spread. Importantly, this is a cold-tolerant species helping it grow well, even at high altitudes. Because this plant has spread out at a large scale in regions of the United States where the temperature is above 8℃, it would be highly possible to spread over the entire Korean peninsula if introduced.
Weed risk assessment
By the result of weed risk assessment criteria (Animal and Plant Quarantine Agency in Korea), degree of risk is 57.4% (Table 2). We suggest that this species needs to be registered to control temporarily in law and to investigate the potential risk.
Brachiaria mutica (Forsskål) Stapf in Prain, Fl. Trop. Africa. 9: 526 (1919)
Korean Name : Africa-gi-jang (아프리카기장; National Institute of Ecology, 2019)
Common Name : Para grass, Buffalo grass, Mauritius signal grass, Pasto pare, Malojilla, Gramalote, Parana, Carib grass, Scotch grass, California grass.
Taxonomic description and distribution characteristics
Taxonomic description Perennial grass with creeping stolons growing up to 5m, vigorous, semi-prostrate. Culms stout, rooting freely from lower nodes, ascending to 2m, 5~8 ㎜ in diameter, nodes densely villous. Leaf sheaths villose or glabrous; leaf blades broadly linear, 10~30 ㎝ long, 1~2 ㎝ wide, pilose or sub-glabrous; ligule membranous, 1~1.3 ㎜ in height. Inflorescence 7~30 ㎝ long, panicle with racemes; racemes 10~20, 5~15 ㎝ long; rachis narrow, winged, scabrous, tinged with purple; pedicels usually setose; spikelets paired or single in upper part of raceme, elliptic, greenish or purplish, 2.5~3.5 ㎜ long, glabrous, acute; lower glume triangular, 1/4~1/3 of spikelet length, 1-veined; upper glume 5-veined; upper lemma rugulose, apex obtuse. Flowers and fruits in August to November, flowers in September to December at Florida, April to May at northern Australia. Although many flower grow, only a few viable seeds are produced, and propagation is usually by vegetative means. Chromosome number 2n=36.
Growing conditions This species covers densely along streams and in other wet places. Sometimes forms a floating rafts.
Origin Northern and central Africa and parts of the Middle East.
Invaded areas This species was naturalized in Australia, US (Florida, Hawaii), the Philippines, Southern Asia and Pacific Islands. This species may have been introduced to the Americas on slave ships, on which it was used for bedding. It was in South America by the early 1800s and Mexico by 1872. It was introduced to Florida by the late 1870s to be used as fodder. It has since escaped cultivation in many areas and it now grows as a widespread weed. It is sensitive to frost so it generally does not persist outside warm regions (Stone, 2010).
Current state of designations This species is an invasive species in many Pacific Islands and Pacific Rim countries. In 1977, this species was listed as a serious weed in Australia, Fiji and Thailand, as a weed in Sri Lanka, Colombia, Hawaii, Jamaica, Malaysia, Peru, the Philippines, Puerto Rico and Trinidad, and as a common weed in Borneo and Mauritius (Holm et al., 1977).
Specimens observed
[Carnegie Museum Herbarium] 258393 (C. E. Devol, 1975. 11. 20. China) ; 222914 (M. L. Briton, 1983. Cuba) ; 222913 (Nassau, India) [United States National Herbarium] 3468738 (Balderrama J. 1994. 8. 3. Bolivia) ; 2977009 (Gerrit Davidse, 1971. 11. 15. Venezuela) ; 1911875 (Gerrit Davidse, 1971. 11. 15. Colombia)
Key compared with morphologically similar species of related Korean genera (Korean name)
1. Spikelets is arranged densely tufted, palea of upper florets has a recurved apex....................... Echinochloa(돌피속)
1. Spikelets is arranged in two rows, palea of upper florets has a enclosed apex with lemma........................................2
2. There is basal swelling on the base of spikelet, lower glume is vestigial...............................Eriochloa villosa(나도개피)
2. There is no basal swelling on the base of spikelet, lower glume is present................Brachiaria mutica(아프리카기장)
Characterisitics of weeds
This perennial plant is native to central Africa, northern Africa and the Middle East and typically thrives in humid regions (e.g., swamps, wetlands). Brachiaria mutica is known to have been introduced to the US as a result of its use as a blanket for slave ships; in other regions, this species has been cultivated as a pastoral plant, facilitating its spread to other regions (Fig. 7). Seeds are dispersed by mixing them with other grains and can also be spread by excretion after eaten by cattle. In addition to reproduction via seeds, plants of this species can grow from broken stem segments capable of producing new roots at their nodes. It reproduces as well through its stolons as through its seeds. Since it is known to be distributed in areas between 15℃ and 22℃ and with annual precipitation between 1,200 ㎜ and 4,000 ㎜, spread of this species could be limited to Jeju Island and southern parts of the Korean peninsula following a theoretical introduction.
Weed risk assessment
By the result of weed risk assessment criteria (Animal and Plant Quarantine Agency in Korea), degree of risk is 36.1% (Table 2). We suggest that this species could be treated as an ordinary weed.
Vulpia bromoides (L.) Gray Nat. Arr. Brit. Pl. ii. 124 (1821)
Korean Name : Deul-muk-sae-a-je-bi (들묵새아재비; National Institute of Ecology, 2019)
Common Name : Barren fescue, Brome fescue, Brome six-weeks grass, Desert fescue, European foxtail fescue, Fall panic grass, Fescue grass, Hair grass, Silk grass, Silver grass, Squirrel-tail fescue.
Taxonomic description and distribution characteristics
Taxonomic description Annual, loosely tufted. Culms solitary or caespitose, erect or decumbent, 5~40 ㎝ long, occasionally up to 60 ㎝ long. Leaf sheaths without keel, glabrous or sparsely pilose; ligule membranous, 0.2~0.6 ㎜ long. Leaf blades flat of involute, 1~18 ㎝ long, 0.5~3 ㎜ wide, linear or filiform, margins entire, apex attenuate, glabrous or pubescent. Inflorescence panicle, 2~12 ㎝ long, panicle branches 4~13 ㎜ wide; panicle open or contracted, equilateral or nodding, predominantly one-sides in nature; spikelet 6~14 ㎜ long, ca. 4 ㎜ wide, pedicels 1~6 ㎜ long; glumes persistent; lower glumes 2~6 ㎜ long, membranous, without keels, 1-veined, lateral vein absent, apex acute; upper glumes 4.5~10 ㎜, longer than lower glume, same length of adjacent fertile lemma, membranous, without keels, 3-veins, apex attenuate; florets 3~10; fertile lemma lanceolate, 6~9 ㎜ long, 5-veined, surface scaberulous, apex acuminate, 1-awned, principal lemma awn 5~12 ㎜ long; palea same length with lemma, 2-veined; anther 1, 0.3~0.6 ㎜ long; ovary glabrous. Caryopsis with adherent pericarp, glabrous, 3.5~4 ㎜ long. Flowers in May to June (England), October to January (Australia). Fruits in May to July (England), October to February (Australia). Chromosome number 2n=14.
Growing conditions This species seem to prefer dry and depleted glassland, low in nutrients, waste land, disturbed ground, stony river beds (Edgar and Connor, 2010). In Australia they have become a major invasive problem of pastures, where they displace more productive grasses and clovers, and crops, such as wheat.
Origin Eastern Africa, the Azores, the Madeira Islands, the Canary Islands, Europe, the middle-east and western Asia.
Invaded areas This species was widely naturalized in Europe except origin, southern Africa, US (including Hawaii), Canada, Australia and New Zealand.
Current state of designations This species is regarded as an environmental weed in Victoria and Western Australia, but not declared or considered noxious by any state government authorities in Australia. In Canada, This species is one of several species named as threatening the habitat.
Specimens observed
[U. of Texas Herbarium] 00209000 (T. F. Newman, 1966. 5. 1. United States) [Carnegie Museum Herbarium] 236858 (Josephine E. Tilden., 1912. 9. Australia) ; 236780 (J. Wolle, 1840. Italy) ; 236781 (Buker, Germany) [United States National Herbarium] 2487072 (A. A. Bettle, 1942. 4. 9. United States) ; 2487071 (G. L. Stebbins, 1942. 6. 2. United States) ; 3532605 (Peteron P. M., 1994. 3. 17. United States)
Key compared with congeneric and morphologically similar species of related Korean genus (Korean name)
1. Perennial......................................................................................................................................................Festuca(김의털속)
1. Annual or biennia...................................................................................................................................................................2
2. Length of lower glume is smaller than 1/3 of upper glume (florets in spikelet 3~5....................Vulpia myuros(들묵새)
2. Length of lower glume is 1/2 of upper glume (florets in spikelet 3~10)......................Vulpia bromoides(들묵새아재비)
Harmfulness and characterisitics of weeds
This annual plant is native to Africa, Europe, and West Asia which is easily propagated to other regions and is quick to adjust in arable land. It spreads around areas disturbed by human beings such as roadsides, open-air spaces, ranches, and beach areas. This plant blooms in the spring mostly and disperses by short lived seeds with little innate dormancy (germinate readily) that can spread rapidly by wind, water flow, animal hair, clothing, and cars (Fig. 8). Plants form massive clusters of high frequency and density, and they grow competitively with native plants. When this plants infiltrate into the pasture, they are known to reduce the purity of the grass. In North America, they distribute in the area of more than 10℃ of the annual average temperature. We investigated this species in grassland of Texas and North Carolina. Then if it is introduced into the Korean peninsula, it is highly possible to spread out all the parts of Korea.
Weed risk assessment
By the result of weed risk assessment criteria (Animal and Plant Quarantine Agency in Korea), degree of risk is 57.4% (Table 2). We suggest that this species needs to be registered to control temporarily in law and to investigate the degree of potential risk.
Lolium persicum Boissier & Hohennacker in Boissier, Diagn. Pl. Orient., ser. 1. 13: 66 (1854)
Korean Name : Persia-ho-mil-pul (페르시아호밀풀; National Institute of Ecology, 2019)
Common Name : Persian darnel, Persian ryegrass, Ryegrass.
Taxonomic description and distribution characteristics
Taxonomic description Annual or not long-lived perennial. Culms erect or decumbent, tufted, 15~70 ㎝ long, somewhat stout, 3~4 noded, scabrid below inflorescence. Leaf blades flat, 4~20 ㎝ long, 1.5~8 ㎜ wide, adaxial surface scabrid; auricles up to 2 ㎜ or absent; ligule 0.5~2 ㎜ long. Raceme straight, 3~21 ㎝ long, rachis scabrid, 1.4~1.5 ㎜ thick, each spikelets are just as far apart as spikelet length, 10~20 ㎜ long, florets 4~11; rachilla internode ca. 0.5 ㎜ long, slightly spinescent; glumes narrowly lanceolate, 2/3 as long to sub- equaling spikelet, 5~7-veined, apex obtuse or acute; lemmas lanceolate, 6.5~12 ㎜ long, 5-veined, apex acute or attenuate into awn; awn 5~20 ㎜ long, slightly curved; palea equal to or slightly shorter than lemma, ciliate along keels. Caryopsis length 3.5~5 times width. Flowers and fruits in June to July. Chromosome numbers 2n=14.
Growing conditions This species does best in dry soils with adequate spring moisture for germination, also grows well in streamsides, roadsides, mountain slopes.
Origin Temperate Asia and southern tropical Asia, such as China, Central Asia (Afghanistan, Kyrgyzstan, Pakistan, Russia, Tajikistan, Uzbekistan), Southwest Asia.
Invaded areas This species was widely naturalized in Europe including England, northern-, central-, southern America including US and Canada.
Current state of designations This species was listed in <Secondary Noxious, Class 3> in the Canadian Weed Seeds Order, 2016 under the Seeds Act.
Specimens observed
[U. of North Carolina Herbarium] 00098279 (R. White, 2003. 7. 10. United States) [Carnegie Museum Herbarium] 262106 (L. Zohann., 1973. 2. 7. Turkey) [United States National Herbarium] 2010914 (G. W. Shewchuk, 1948. 6. 20. Canada) ; 1537983 (A. N. Pung, 1929 7. 10. United States) [New York Botanical Garden] 1737589 (O. A. Stevens, 1942. 6. 13. United States) ; 1737588 (O. A. Stevens, 1957. 6. 30. United States)
Key compared with Korean congeneric species (Korean name)
1. Upper glume is longer than spikelet, the length of mature caryopsis is less than three times of width.......................
.........................................................................................................................................................Lolium temulentum(독보리)
1. Upper glume is shorter or sub-equal than spikelet, the length of mature caryopsis is three times more than width
........................................................................................................................................................................................................2
2. Annual (or not long-lived perennial), awn in lemma present or absent............................................................................3
3. Florets 3~10, length of glumes is longer than the half length of spikelet.........................................................................4
4. Rachilla is 2~3 ㎜ thick, spikelet partially retracted in rachilla, lemma 5~8 ㎜ long, awn absent, if present, 3~8 ㎜ long in upper florets of inflorescence..............................................................................................Lolium rigidum(댕돌보리)
4. Rachilla is 0.4~1.5 ㎜ thick, spikelet not retracted in rachilla, lemma 9~15 ㎜ long, awn 5~20 ㎜ long, present in all florets ....................................................................................................................................Lolium persicum(페르시아호밀풀)
3. Florets 8~22, length of glumes is shorter than the half length of spikelet..........................Lolium multiflorum(쥐보리)
2. Perennial, awn of lemma absent................................................................................Lolium perenne(호밀풀, 가는보리풀)
Harmfulness and characterisitics of weeds
This perennial herb of temperate and tropical southern Asian origin is distributed in a wide range (i.e., from 50° N to 46° S in latitude). In North America, Lolium persicum has been used for grassland formation, pasturage, soil erosion prevention, and sand dune stabilization. This species produces up to 2,800 seeds per plant and 53,000 seeds/m2 when grown without competition; this leads to rapid spreading to proximal arable land, gardens, trackway, roadside and disturbed areas, thus threatening natural grassland vegetation (Fig. 9). This plant was observed spreading in the grasslands of Texas. This weed competes with plant species for water, light, nutrients, and space and produces chemical compounds called allelochemicals, that affect crops, especially barley. Growth of this species occurs in: i) warm temperate regions with annual precipitation of at least 650 to 700 ㎜, and ii) regions with mild winters and fertile soil, The lowest temperature average is over 0℃, and the optimum growth temperature is 18 to 20℃. For these reasons, if introduced into the Korean peninsula, it is highly possible that this species could spread throughout all parts of Korea.
Weed risk assessment
By the result of weed risk assessment criteria (Animal and Plant Quarantine Agency in Korea), degree of risk is 60.7% (Table 2). We suggest that this species should to be registered to control in law.
Setaria palmifolia (J. Konig) Stapf, J. Linn. Soc., Bot. 42: 186 (1914)
Korean Name : Neol-beun-ip-gang-a-ji-pul (넓은잎강아지풀; National Institute of Ecology, 2019)
Common Name : Palm grass, Highland pitpit, Hailans pitpit, Short pitpit, Broadleaved bristlegrass, Knotroot.
Taxonomic description and distribution characteristics
Taxonomic description Perennial, having a short knotty rhizome. Culms erect or geniculate, 0.75~2 m high, sometimes up to 3m, 3~7 ㎜ in diameter, sometimes up to 10 ㎜. Leaf sheaths usually sparsely hipid, glabrous but margins tuberculate-ciliate near ligule, ligule 2.0~3.5 ㎜ long; leaf blades fusiform-lanceolate, finely pleated with multiple ridge, 20~60 ㎝ long, 2~7 ㎝ wide, sometimes up to 1m long, glabrous or hispid, base narrowed, apex acuminate. Panicles 20~60 ㎝ long, 2~10 ㎝ wide, branches up to 20 ㎝ long, loosely spreading, flexuous, some spikelets subtended by a single 4~15 ㎜ bristle; spikelets broadly lanceolate or oval, 2~4 ㎜ long, apex acute; lower glume triangular-ovate, 1/3~1/2 as long as spikelet, obtuse to acute; upper glume ovate, 1/2–3/4 as long as spikelet, 5–7-veined, acute; lower lemma neuter, often distinctly longer than upper floret, 5-veined, tipped with a short incurved beak; lower palea narrow, hyaline, 2/3 as long as lemma; upper lemma indistinctly rugulose to almost smooth, slightly shiny, apex apiculate, green and compressed. Mature seeds pale brown, persisting, enveloped with glume and lemma, 2 ㎜ long, ovate, slightly fattened. Flowers in August to November (mainly in summer), Fruits in September to December. Chromosome numbers 2n=36, 54 (Bor, 1960; Flora of China Editorial Committee, 2012).
Growing conditions This species grows well in open forests, sub-humid woodlands, semi-arid shrub woodlands, margins of thickets and shady pathsides.
Origin Temperate and tropical Asia (China, southern parts of Japan, Taiwan, Southeast Asia, etc.)
Invaded areas This species has been widely introduced to Central America and Pacific, usually as an ornamental, and has naturalized and become invasive in many new territories, especially on Pacific islands, including Hawaii.
Current state of designations This species can be a serious weed of forestry, plantation crops and of rice, but also threatens endangered species in natural forest and other natural vegetation. Holm et al. (1979) classified it as ‘serious’ in India and Indonesia, while PIER (2012) score it 7 on the Australian weed risk assessment system, meaning that it should not be imported into Australia.
Specimens observed
[Carnegie Museum Herbarium] 364388 (Wang Zhong-tao, 1987. 8. 14. China) ; 496477 (D. B. Rogan, 1997. 5. 6. New Zealand) ; 293430 (G. Davidse, 1977. 10. 1. Nicaragua) ; 239413 (E. Fenix, 1910. 11. Philippine) [New York Botanical Garden] 00194989 (C. R. Annable C., 1996. 4. 5. United States) ; 01815900 (L. F. Muller, 1853. Mexico) ; 01815899 (L. F. Muller, 1855. Mexico)
Key compared with Korean congeneric species (Korean name)
1. Leaf blades flatted, not plicate, base rounded, 0.5~2 ㎝ wide. branches of panicle distinctly spreading while maturing.....................................................................................................................................Setaria chondrachne(조아재비)
1. Leaf blades flatted, but plicate, base become narrow, 2~7 ㎝ wide. branches of panicle not distinctly spreading while maturing.....................................................................................................................Setaria palmifolia(넓은잎강아지풀)
Harmfulness and characteristics of weeds
A perennial herb of temperate and tropical Asian origin, Setaria palmifolia grows well in warm, well-drained climates and is widely distributed in broad latitudes. Because of the corrugated features common in palm trees, these plants are cultivated for ornamental use, some of which make various stripes on the leaves through improvement, and others for grain used as a substitute for rice, medicinal, and feed pastures (Fig. 10). Plants of this species spread rapidly through seeds which can be spread by wind, animals and machinery. This plant can grow in semi-shade (light woodland) or no shade and prefers moist soil and can tolerate drought and strong winds, but not maritime exposure. Setaria palmifolia usually grows well on disturbed waterways, riversides, roadside, gardens, vacant lots, etc. and it also invades wet forest areas, grasslands and open forests. This species was observed in a forest on the grounds of the University of Florida, Gainsville and it was noted that several individuals planted for testing spread rapidly to surrounding areas. Reproduction of this species may be difficult under 5℃ because of subtropical tropical natives, however, if the roots do not freeze, the aerial parts will grow in the Spring. It is possible that this species would spread to regions below 600 meters on Jeju island if introduced.
Weed risk assessment
By the result of weed risk assessment criteria (Animal and Plant Quarantine Agency in Korea), degree of risk is 54.1% (Table 2). We suggest that this species needs to be registered to control temporarily in law and to investigate the degree of potential risk.
Prosopis glandulosa Torr. in Ann. Lyc. N.Y. 2:192 (1827)
Korean Name : Yang-me-tu-gi-kong (왕메뚜기콩; National Institute of Ecology, 2019)
Common Name : Honey mesquite.
Taxonomic description and distribution characteristics
Taxonomic description Deciduous tree, 6~9m tall, in some cases up to 15m tall, outward appearance round because of big and drooping branches. Thorns stout, axillary, 0.7~5 ㎝ long. Leaves alternate, bipinnate; rachis 2.5~12.5 ㎝ long, prolonged beyond the last pinnae as a soft bristle, base swollen and glandular, pinnae 1~2 pairs, 6~17 ㎝ long, sometimes glandular between the leaflets; leaflets subsessile, 7~18 pairs, 1.5~4 ㎝ long, 1.5~5 ㎝ wide, linear to oblong, apex acute to obtuse, glabrous, subcoriaceous. Inflorescence axillary, pedunculate spikes or racemes, racemes 5~14 ㎝ long, multiflorous, flowers yellow; peduncles 5~18 ㎝ long. Calyx campanulate, 1~2 ㎜ long, 5-toothed, teeth ciliate or glabrous; petal 3.4~4 ㎝ long, oblong, hairy within towards the tips. Flowers in March to November (mainly April to August), Fruits in March to November. Pods of legume yellowish, 5~20 ㎝ long, 7~8 ㎜ wide, linear or linear falcate, compressed, turgid, pendulous, narrowed into a short stalk, exocarp coriaceous, mesocarp pulpy, endocarp cartilaginous. Seeds 5~20. Chromosome numbers 2n=28, 56, 112.
Growing conditions This species has a broad ecological amplitude and is adapted to a very wide range of soils and site types from sand dunes to cracking clays. And this species dominates in dry, or seasonally dry, watercourses or depressions, and the presence and depth of the water table is a decisive factor in the distribution, size and growth. The height of this trees has been used successfully to estimate the depth of the water table in the USA (Simpson, 1977).
Origin Southern United States, Northern Mexico and North America.
Invaded areas This species was naturalized in Pakistan, Australia, etc.
Current state of designations Because of this species infamy as an invasive species, several governments have banned the importation of seed. This species is a declared noxious weed in Australia and South Africa, and the genus as a whole is regulated in several other countries.
Specimens observed
[U. of Florida Herbarium] 128784 (John H. Belsik, 1968. 4. 14. United States) ; 13414 (R. A. Knight, 1934. 5. 21. United States) ; 128126 (Marc S. Frank, 2005. 2. 3. United States) ; 150292 (S. L. Hatch, 1981. 6. 31. United States) [Arizona State Univ. Herbarium] 0064278 (T. R. Van Devender, 2010. 5. 17. Mexico) ; 0024696 (T. R. Van Devender, 1992. 6. 3. Mexico) ; 0024674 (Donald J. Pinkava, 1967. 8. 12. Mexico) [Carnegie Museum Herbarium] 376994 (Chris Sherman, 1991. 9. 11. United States) ; 469498 (Binnie L. Issac., 2005. 8. 12. United States)
Key compared with morphologically similar species of related Korean genera (Korean name)
1. Tree climbing; (leafles 5~10 paired; pods oblong, below 10 ㎝ long)....................Caesalpinia decapelata(실거리나무)
1. Tree erect................................................................................................................................................................................2.
2. leaflets 5~7 paired; pods linear, twisted, ca. 23 ㎝ long..............................Gleditsia japonica var. koraiensis(주엽나무)
2. leaflets 8~18 paired; pods linear, falcate or straight, constricted between seeds, 12~20 ㎝ long..................................
..................................................................................................................................................Prosopis glandulosa(왕메뚜기콩)
Harmfulness and characterisitics of weeds
This deciduous tree is native to the southern regions of the United States and northern Mexico and grows on riversides, estuary wetlands, and roadsides with waterways. There are a wide range of uses of this species for medicinal, food, building materials, fuel, and honey, and seeds are edible (Fig. 11). In a southern California study, this tree had an average of 100 pods per tree, 12 seeds per pod, of which an average of 5 were destroyed by insects. It is known to reduce the plant diversity affecting the damage to the existing native wetland plants by invading into the slopes and wastelands of sand dunes. Although adapted to most soil types, in Texas, honey mesquite tends to grow best on medium to fine-textured soils, but overall, it grows well in areas: i) 50 to 1,200 meters above sea level, ii) an average temperature of 20 to 30℃, and iii) an average precipitation of 50 to 1,200 ㎜. This species could be grown in central and southern regions of Korea if introduced, however, based on its natural habitat, it will not likely spread efficiently.
Weed risk assessment
By the result of weed risk assessment criteria (Animal and Plant Quarantine Agency in Korea), degree of risk is 41.0% (Table 2). We suggest that this species could be treated as an ordinary weed.
Fallopia baldschuanica (Regel) Hobub. in Folia Geobot. Phytotax. 6:176 (1971)
Korean Name : Jung-guk-dal-geu-deong-gul (중국닭의덩굴; Ministry of Environment and National Institute of Ecology, 2014)
Common Name : Silver lace vine, Russian-vine, Mile-a- minute-vine, Bukhara fleece flower, Chinese fleece vine, Chinese bindweed.
Taxonomic description and distribution characteristics
Taxonomic description Perennial herb. Stem long up to 10m, usually twisting, glabrous, scandent on trees, the lower part woody, barks brownish gray, with numerous lenticels, young branches angled with prominent ribs, small glands on ribs. Leaf sheaths deciduous, pellucid membranous or brownish, cylindric, appressed to stem, glabrous, 3~8 ㎜ long; petiole 2~4 ㎝ long; leaf blades oblong-ovate, 2.5~10 ㎝ long, 1.5~5.5 ㎝ wide, base cordate, margin obsoletely crenulate, apex obuse to acuminate. Inflorescence paniculate, up to 50 ㎝ long, slender, drooping or spreading; pedicel 4-12 ㎜ long, jointed at the middle or nearer the base; flowers 5-8 ㎜ in diameter, bright pink, turning reddish; stamens 6~8, filaments pubescent in lower part; perianth deeply partite, with a decurrent base; bracts oval, acute, ca. 2 ㎜ long, fruiting perianth obovate or obcordate, ca. 12 ㎜ long, ca. 10 ㎜ wide, white or roseate, wings ca. 3 ㎜ broad. Fruits 2~4 ㎜ long, 1.8~2.2 ㎜ wide, acute with strongly concave faces, rather dull dark brown. Flowers in August to September, Fruits in August to October. Chromosome number 2n=20.
Growing conditions This species is found growing on fences, hillsides, trees and on other vegetation in a variety of habitats. It tolerates a wide range of soil conditions and is most abundant in full sun to part shade.
Origin This species is native to Central Asia (Pamir Alai, Pakistan, Russia, etc.)
Invaded areas This species was naturalized in Central America, Northern America and Europe, etc.
Current state of designations This species is classified as a Weed of Concern by the King County Noxious Weed Control Board and is not on the Washington State Noxious Weed List.
Specimens observed
[U. of North Carolina Herbarium] 00076629 (Allison Shaw, 2006. 8. 30. United States) [U. of Texas Herbarium] 00377612 (Lloyd H. Shinners, 1947. 10. 11. United States) [United States National Herbarium] 1654343 (E. B. Copeland, 1931. 6. 1. United States)
Key compared with Korean similar species (Korean name)
1. Annual; Inflorescence raceme................................................................................................................................................2
2. Outer 3 perianth keeled or almost not winged (achene 3~3.5 ㎜ long)...................Fallopia convolvulus(나도닭의덩굴)
2. Outer 3 perianth winged.........................................................................................................................................................3
3. wings dentate or rugose; achene 1 ㎝ long, minutely granular striate......................Fallopia dentatoalata(큰닭의덩굴)
3. wings entire; achene 5~7 ㎜ long, smooth.............................................................................Fallopia dumetora(닭의덩굴)
1. Perennial, stem woody or not, Inflorescence panicle..........................................................................................................4
4. Stem not woody; achene 7~8 ㎜ long....................................................................................................................................5
5. Leaf glabrous on both surface.....................................................................................................Fallopia multiflora(하수오)
5. Veins on abaxial surface pilose or papillose........................................................................Fallopia ciliinervis(나도하수오)
4. Stem woody; achene 2~4 ㎜ long.............................................................................Fallopia baldschuanica(중국닭의덩굴)
Harmfulness and characterisitics of weeds
A perennial climber native to Central Asia, this plant grows very fast and is widely used for soil stabilization and to cover ugly walls, other structures, and old stumps. Fallopia baldschuanica has also been conveniently cultivated as an aromatic or garden plant, which is assumed to be capable of spreading to surrounding areas. Plants of this species have an energetic rhizome system and the potential to grow from rhizome and stem fragments (Fig. 12). The ideal growth conditions are well-drained sandy loams with regular moisture and full sun to partial shade. There is tolerance for a wide range of soil conditions, including some drought. A hybrid with Reynoutria japonica Houtt. (=Fallopia japonica (Houtt.) Ronse Decr., 호장근) has been recorded in and described from the British Isles. Fallopia baldschuanica is widely spread on roadsides, fences, landscaping sites and vacant lots where it has been disturbed by humans; once it has spread, it can defoliate and kill indigenous plants by covering them. As plants of this species are distributed to regions with an annual average temperature of 16-20℃, it likely could spread to South and Central Korea and Jeju Island if introduced.
Weed risk assessment
By the result of weed risk assessment criteria (Animal and Plant Quarantine Agency in Korea), degree of risk is 47.5% (Table 2). We suggest that this species could be treated as an ordinary weed.