Introduction
Materials and Methods
Crop cultivation and soil conditions of experimental field
Measurement of growth and development characteristics of root
Constituent of major and minor mineral nutrients, inorganic substance
Analysis of major and minor mineral nutrients
Analysis of constituent amino acid
Statistical analysis
Results and Discussion
Condition of soil on cultivation field
Root growth by soil moisture content
Ingredient contents by soil moisture content
Introduction
It is well known that light, air temperature, air, soil texture and moisture are major limiting factors in crop production (Henderson et al., 2018; Jeong et al., 2020; Lee et al., 2010b, 2000). Soil moisture act a limiting factor in plant growth and development, and more than 50% content of soil induce severe reduction in crop productivity on upland fields (Henderson et al., 2018; Khalid, 2006; Lee et al., 2008; Lee et al., 1999; Osakabe et al., 2014). Moreover, water treatment influence not only growth but also chemical composition of plants (Khalid, 2006).
Soil moisture sensor-based systems are promising, however, there is not enough information on crop type, or field condition (Kang et al., 2020; 2021). Recently development of sensing technology and data analysis technique leads to automation of agronomy labors, and data based agriculture is enriched through cloud technology (Henderson et al., 2018; Kim et al., 2021). So far, limited automatic irrigation system that controlled and warned about under- and overwatering message is partly used in open field agriculture (Kang et al., 2020; Henderson et al., 2018).
As a well-known medicine food homology species, Platycodon grandiflorum (PG) has various pharmacological effects and health benefits, and is widely distributed in China, Korea, Japan and eastern Siberia (Huang et al., 2021; Lee et al., 2020). PG contains proteins, lipids, sugars, ash, iron, saponin, inulin and phytosterin. It contains multiple essential nutrients, including proteins, starches, vitamins, minerals and amino acids, and has been widely consumed as a dietary supplement for its various beneficial effects. Moreover, it has various pharmacological actions, such as expectorant, antitussive, antibacterial, hypotensive and hypoglycemic effects (Choi and Lee, 2018; Lee et al., 2010a; 2010b; Zhang et al., 2022). As an important traditional herbal medicine, its root is used to treat several diseases including, hyperlipidemia, hypertension, and diabetes (Lee et al., 2010a, 2010b; Zhang et al., 2022). The therapeutic effects of PG will be exerted in more than 2 years cultivated plants, and thus over two-year-old PG roots were used as traditional herbal medicine to relieve cough, excessive phlegm, sore throat, tonsillitis, chest congestion and other pulmonary or respiratory ailments.
The multiple chemical compositions and pharmacological activities of PG have been broadly investigated in the past decades (Huang et al., 2021; Lee et al., 2020; Wang et al., 2017; Yan et al., 2013; Zhang et al., 2022). In recent decades, the biological activities of PG, including its anti-tumor, hepatoprotective, immunoregulatory and anti-oxidant effects, have resulted in the compositions of saponins, flavonoids, anthocyanins, phenolics, and polysaccharides, among other compounds from the plant (Huang et al., 2021; Lee et al., 2020; Zhang et al., 2022).
The data for the growth characters and components of P. grandiflorum roots by the soil moisture conditions were investigated in this study using an automatic irrigation system that has constructed by our study (Lee et al., 2021).
Materials and Methods
Crop cultivation and soil conditions of experimental field
Seedlings of one-year-old bellflowers (Platycodon grandiflorum) with 5 to 6 leaves were purchased from bellflower plantation (Bellflower Farm, South Korea) and used for cultivation. The one-year-old bellflower seedlings were transplanted to farmland fields which is controlled by the soil moisture control system (AcroTNS Com., South Korea) on May 26th, 2021 (Lee et al., 2021). The soil components used in this experiment were shown by Lee et al. (2021). The farmland was mulched with black vinyl film with 100 ㎝ in width. All agricultural practices, other than the experimental treatments, were done according to the recommendation of the RDA, Korea (Rural Development Administration, 2012).
Soil moisture sensing was executed by the sensors (WT1000B and WT1000A, MiraeSensor Com., South Korea) on the surface of soil with 6 ㎝ to 11.5 ㎝ in depth (Lee et al., 2021). Moreover, EC (Electric conductivity) and soil temperature were also checked. Soil moisture was controlled by 20%, 30%, 40% and 50%, respectively, according to manual of AcroTNS Company (AcroTNS Com., South Korea). Soil moisture was monitored and recorded using moisture sensor plugged into data loggers at 2-min intervals over the entire growth cycle (Lee et al., 2021). Sensors were inserted into the substrate from the top, with sensor prongs close to the center of the ridge (Henderson et al., 2018; Lee et al., 2021).
Measurement of growth and development characteristics of root
After full maturity, all plants of each plot harvested and measured for determining below ground growth characteristics, such as root length, root diameter, fresh weight and dry weight (Kwon et al., 2019; Rural Development Administration, 2012).
Constituent of major and minor mineral nutrients, inorganic substance
Reagents : HNO3 and H2O2 for CRM hydrolysis were used EP-S (electronic grade, DongWooFineChem Company, Korea). Ca and Na standards (1,000 ㎎/L) were purchased from AccuStandard company (U.S.A). Distilled water was deionized by equipment (MILLIPORE, Milli-Q, USA) and used for analysis. All other chemicals were used as analytical reagent grade. Equipment for microwave digestion were Multiwave PRO (Anton Paar, USA). ICP-AES Avio 500 (Perkin Elmer, USA) was used for measurement of Ca and Na.
Analysis of major and minor mineral nutrients
One ㎎ of samples were weighing and set on Tefron beaker for disassemble by microwave digestion. And then added 8 ㎖ HNO3 and 2 ㎖ H2O2. Samples were hydrolysed and 50 g of sample solution was mixed. The sample solution was used for analysis. Minerals were analyzed by ICP-AES (L-8900, Hitachi High Tech, Tokyo, Japan). The conditions for analyses of minerals by ICP-AES (L-8900, Hitachi High Tech, Tokyo, Japan) were shown in Table 1.
Table 1.
Conditions for analyses of minerals by ICP-AES
| Instrument | ICP-AES, Avio 500, Perkein Elmer | |
| Power (W) | 1500 | |
| Use Gas | Argon gas | |
| Pump Speed (rpm) | 2.50 | |
| Nebulizer Flow (L/min) | 0.7 | |
| Wavelength (㎚) |
Na Ca |
589.592 317.933 |
Analysis of constituent amino acid
Constituent amino acids in Platycodon grandifflorum were analyzed by acid hydrolysis method. The sample, 0.2 g to 0.5 g, was thoroughly mixed with 3 ㎖ of 6 N-HCl in a test tube and tightly sealed. The mixture was subjected to acid hydrolysis in a heating block (Thermo-Fisher Scientific Co., Rockford, IL, USA) at 105°C for 22 hr. The hydrolyzed sample, 10 ul, and sodium dilution buffer (pH 2.2), 990 ㎕, were mixed and subsequently filtered through a 0.2 ㎛ PTFE membrane filter. An amino acid analyzer (L-8900, Hitachi High Tech, Tokyo, Japan) was used to determine the profiles of constituent amino acids. The conditions for analyses of amino acids by ICP-AES (L-8900, Hitachi High Tech, Tokyo, Japan) were shown in Table 2.
Table 2.
Conditions for analyses of amino acids by ICP-AES
Statistical analysis
All of the experiments were executed with three (ingredient content) to five (plant-growth factors) replications. Using the SAS program (SAS, 9.2, Institute Inc, USA), statistical analysis was conducted by Duncan’s multiple range test (DMRT, p=0.05). Frequency and percentage were used to analysis the qualitative characters, whereas mean and standard deviation were used for quantitative data analysis (Lee et al., 2021; 2022).
Results and Discussion
Condition of soil on cultivation field
Monitoring for the soil moisture content, EC and soil temperature were executed on the base of automatic soil moisture content control system under farmland conditions and the trend of environmental data were reported by Lee et al. (2021, 2022). In addition, the trends of environmental data on farmland were checked after cultivation of plants in the field and shown in Table 3.
Table 3.
Characteristics of soil components of the cultivation field after the plant culture at Sunchon National University (SCNU), South Korea
Item Site |
pH (1:5) |
Organic Matter (g/㎏) |
P (㎎/㎏) |
K (c㏖+/㎏) |
Ca (c㏖+/㎏) |
Mg (c㏖+/㎏) |
Electric Conductivity (dS/m) |
| Az | 5.7 | 28 | 513 | 1.07 | 6.0 | 2.1 | 0.7 |
| By | 5.9 | 19 | 351 | 0.91 | 6.7 | 2.7 | 0.8 |
| Cx | 5.9 | 14 | 242 | 0.85 | 7.6 | 2.6 | 0.4 |
| Mean | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Optimal Range | 6.0~7.0 | 20~30 | 300~550 | 0.5~0.8 | 5.0~6.0 | 1.5~2.0 | < 2 |
On the base of automatic soil moisture control system, the soil moisture was controlled from 20%, 30%, 40% and 50%, respectively (Lee et al., 2021), and the moisture contents were measured from May to December, 2021 (Lee et al., 2022). The soil moisture content was controlled by the system showing over the controlled-soil moisture contents except 40% treatment. The 40% treatment showed 30% in the moisture which is lower than that of the system controlled. Lee et al. (2021) assumed that the reason showing the higher soil content might be resulted from differences by rainfall, drainage and soil condition such as, uniformity of soil textures. And the lower soil content might be resulted from operating of automatic irrigation system or insertion position of sensor on the surface of farmland fields. Therefore, the position of sensors at the ridges is very important when moisture content is monitored on open farmland condition (Henderson et al., 2018; Kang et al., 2020; 2021).
As shown Fig. 1, additional data for the soil moisture content, EC and soil temperature were measured for 1 month, March, 2022. As a result of monitoring of the soil moisture by using automatic irrigation control system, even though the sensing data were not matched directly, the system was worked properly (Lee et al., 2021; 2022). Thus, we sampled roots for growth and ingredient contents analyses on 20% and 50% moisture content, respectively.
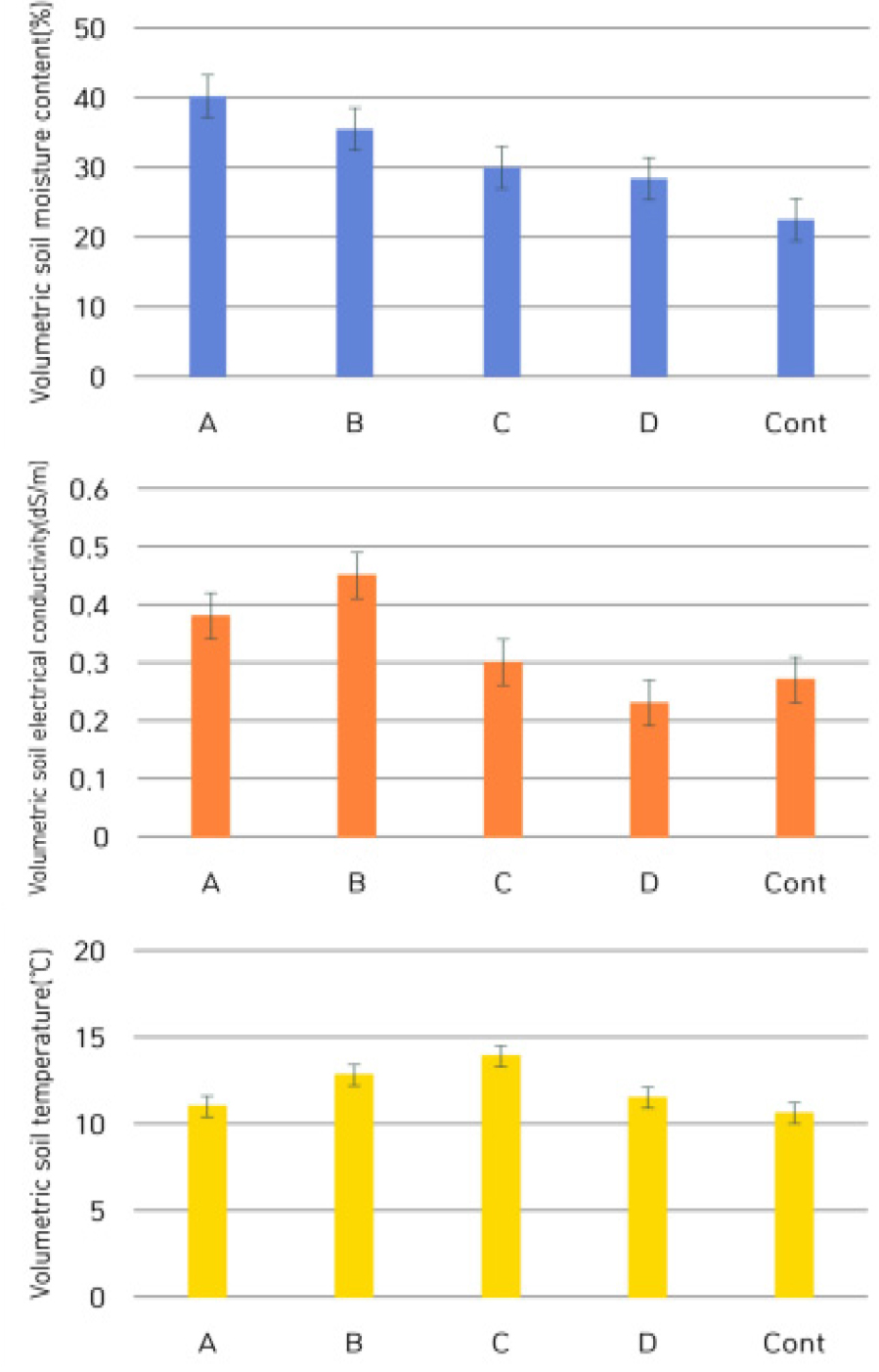
Fig. 1.
A month (March, 2022) soil moisture (upper), EC (middle) and soil temperature (bottom) at the field condition. *Upper: Soil moisture contents were set as A: 50%, B: 40%, C: 30%, D: 20% by the soil moisture control system with soil sensing and automatic water supply chain, and control was shown at 22.5% on the field condition, respectively.
Root growth by soil moisture content
Growth of two-year-old roots by soil moisture contents in bellflower plants were shown in Table 4 and Fig. 2. Root diameter, root number and root length of the plants were significantly influenced by water treatment with 20%, 30%, 40% and 50%, respectively. Moreover, fresh weight and dry weight of the plant roots were significantly influenced by water treatment, 20%, 30%, 40% and 50%, respectively. In detail, main root and fine root diameter, main root and root number have a tendency that showing more increase from 20% to 40% of the water contents compared with control, and those were decreased in 50% of the water content. Especially, fine root numbers were decreased in 50% treatment (Fig. 2). Also, fresh weight, dry weight, shoot fresh weight and shoot dry weight have showing increasing by the water content from 20% to 40% compared with control, and these were decreased in 50% of the water content.
Table 4.
Characteristics of growth of roots of bellflower (Platycodongrandiflorum) by soil moisture treatment with cultivation of two-year-old tuberous roots
In general, approximately 50% of soil moisture is a maximum content for normal plant growth and development (Henderson et al., 2018; Khalid, 2006; Lee et al., 2008; Lee et al., 1999; Osakabe et al., 2014). However, no significant decrease in all growth factors in this study. This may be resulted from mismatching between sensor controlled data and the field data (Lee et al., 2021). The result means higher soil moisture act on repressor plant growth, and it is consistent with other crop plants (Kang et al., 2021; Khalid, 2006). Especially, Henderson et al. (2018) reported that approximately 50% of soil moisture is a maximum content for a normal plant growth and development and it leads severe reduction for crop productivity on upland fields.
Ingredient contents by soil moisture content
Ingredient contents of two-year-old roots in bellflower plants were detected in the 20% and 50% controlled soil moisture content.
Mineral nutrients
The mineral nutrients contents by soil moisture contents in two-year-old roots of bellflower plants were shown in Table 5. Phosphorus content of the root was 140.5 ㎎/100 g in control, and that of treatment was significantly decreased in the soil moisture treatment with 20% and 50% showing 112.2 ㎎/100 g and 120.4 ㎎/100, respectively. However, except for phosphorus nothing shown significant decrease in mineral contents. Potassium content of the root was 475.8 ㎎/100 g in control, and that of treatments was decreased in 20% and 50% soil moisture showing 401.3 ㎎/100 g and 423.4 ㎎/100, respectively. Magnesium content of the roots was not showed difference by soil moisture contents compared with the control. Sodium, none-essential nutrient element, content was somewhat higher (11.3 ㎎/100 g) in the 50% soil moisture treatment compared with the control (9.5 ㎎/100 g). Contents of minor nutrients, iron and zinc, were showed few difference by the soil moisture treatment.
Table 5.
Mineral contents of roots of bellflower (Platycodon grandiflorum) by soil moisture treatment with cultivation of two-year-old tuberous root (㎎/100 g)
Chemical composition in bellflower was reported in leaves and stems of (Jeong and Shim, 2006), roots (Lee et al., 2013) and raw ginseng (Kim et al., 2002). The composition was different by genotype (Yan et al., 2013), and cultivation year and soil texture (Lee et al., 2000) of PG, and plant organs and cultivation year of roots in Panax ginseng C,A. Meyer (Park et al., 2012). In addition, it was reported main components contents were different according to grow period in Codonopsis lanceolata (Im et al., 2021). In this study, some of minerals were different by soil moisture contents.
Amino acid
Amino acid contents of two-year-old roots of bellflower plants were shown in Table 6. Showing no difference in proline and tyrosine, all of the amino acid contents were gradually decreased by increased soil moisture contents, with significant decrease in serine, glycine, alanine, leucine, lysine and histidine at 20% treatment.
Table 6.
Amino acid contents of radix of bellflower (Platycodongrandiflorum) by soil moisture treatment with cultivation of two-year-old tuberous root (㎎/100 g)
Treatment Amino acids | CONT* | 20% | 50% |
| Asp | 179.4±31.75ay | 133.2±10.45a | 168.2±28.6a |
| Thrz | 97.8±15.81a | 68.0±7.78a | 68.0±7.78a |
| Ser | 95.1±15.59a | 69.9±69.87b | 82.9±6.53ab |
| Glu | 1301.5±495.72a | 780.6±206.99a | 1139.8±41.58b |
| Pro | 17.1±7.26a | 21.3±5.72a | 16.0±4.44a |
| Gly | 64.3±3.03a | 51.4±1.29b | 59.8±5.03a |
| Ala | 81.5±5.98a | 59.4±7.37b | 74.6±5.66a |
| Valz | 86.9±12.12a | 66.5±6.07a | 80.1±18.45a |
| Metz | 20.7±11.06a | 11.2±3.50a | 13.4±2.85a |
| Ilez | 60.8±6.22a | 46.6±5.39a | 57.1±12.63a |
| Leuz | 98.7±9.06a | 80.0±3.84b | 94.8±12.00ab |
| Tyr | 52.2±5.75a | 53.3±9.53a | 52.3±11.67a |
| Phez | 72.0±4.75a | 63.5±9.64a | 70.8±7.66a |
| Lysz | 103.6±5.50a | 81.2±1.73b | 96.3±9.77a |
| Hisz | 57.5±7.55a | 43.2±2.89b | 49.9±7.72ab |
| Arg | 1025.7±141.58a | 910.8±96.85a | 886.6±267.89a |
| Total A.A | 3414.8 | 2540.1 | 3010.6 |
| Total E.A.A1) | 598 | 460.2 | 530.4 |
Total amino acid contents of the roots were 3,414.8 ㎎/100 g in control, and the contents of treatment were decreased in the soil moisture with 20% and 50% showing 2,540.1 ㎎/100 g and 3,010.6 ㎎/100 g, respectively. Essential amino acid contents of the root were significantly decreased by the treatment of soil moisture showing 598.0 ㎎/100 g, 460.2 ㎎/100g and 530.4 ㎎/100 g in the control, 20% and 50% treatment, respectively. In addition, the content of amino acid was the highest in glutamic acid, followed by arginine, and the proline was the lowest at control. And the content of amino acid was the highest in arginine, followed by glutamic acid, and the methionine was the lowest at 20% and 50% treatments.
Under water stress condition in paddy field, the main constituents of essential oil, proline and total carbohydrate content increased, and N, P, K, and protein decreased in ginseng (Khalid, 2006). As a heat shock, roasting induced diverse patterns of amino acid profiles in PG roots (Lee et al., 2020). In this study, water content in soil affected to amino acid patterns, also. Jeong and Shim (2006) reported difference of amino acid contents between leaf and stem in ginseng showing glutamic acid, followed by arginine in leaf, lysine, followed by glutamic acid in stem parts of genseng plant. Moreover, their data shown that the amino acid contents were different from cultivation year, and the contents were high in glutamic acid, followed by arginine in 3-year cultivated roots, and in arginine, followed by alanine in 24-year cultivated roots.





