Introduction
Materials and Methods
Plant material and sampling procedure
Analytical procedures and data analysis
Statistical analysis
Results and Discussion
Morphological changes due to seasonal variations
Sloughing-off process in summer-harvested roots
Seasonal variation in rubber production of Russian dandelion T. kok-saghyz
Introduction
Taraxacum kok-saghyz L.E.Rodin is a rubber-producing dandelion native to Kazakhstan and parts of Russia. Natural rubber from T. kok-saghyz is essentially similar to that of the rubber tree Hevea brasiliensis (Cherian et al., 2019). Worries about skyrocketing petroleum prices and diseases in Hevea trees have ignited renewed interest in alternative rubber sources.
Changing weather patterns in Southeast Asia and a shortage of land are also of great concern in the rubber industry. Thus, there is an urgent need for the development of alternative natural rubber sources. Developing T. kok-saghyz as a commercial rubber source could offer a positive solution to this problem. Russian dandelion contains 4%-8% high-quality rubber, produced in laticifers accompanying the vascular bundles throughout the plant (Kekwick, 2001). Tires made from Russian dandelion rubber were as resilient as those made from H. brasiliensis, and more resilient than guayule-rubber-based tires (Heim, 2003). The Russian dandelion also produces another useful substance; 25% to 40% of its dry root weight is composed of inulin, which has several applications in the food industry (Eggert et al., 2018).
At present, T. kok-saghyz is commercially grown and processed but has not yet become common in the market. One reason for this is that the percentage of rubber content in T. kok-saghyz is not sufficient for commercial exploitation, and another is the lack of an economical method of rubber extraction. Other questions that need to be answered before the commercialization of T. kok-saghyz are whether and to what extent rubber content varies with seasonal variation. An understanding of the changes in rubber content over the growth cycle can help us determine the best time for harvesting T. kok-saghyz.
Seasonal variations in rubber biosynthesis have been studied in an alternative source of rubber, guayule (Parthenium argentatum A. Gray) (Ji et al., 1993; Schloman Jr. et al., 1986; Stonebloom and Scheller, 2019). However, few studies have reported the seasonal variation of rubber content in T. kok-saghyz. Seasonal patterns of biomass, rubber, and inulin content have been reported for T. kok-saghyz grown in German experimental fields (Kreuzberger et al., 2016). Seasonal morphological changes in the T. kok-saghyz plants and the loss of the outer layer of roots along with coagulated roots have also been reported (Kreuzberger et al., 2016). Annual shedding of the root bark of T. kok-saghyz in the form of solid rubber threads in April and May has been reported (Buranov and Elmuradov, 2010). Simulation of approximate summer and winter temperature regimes in guayule’s natural environment has been conducted to elucidate the induction of rubber biosynthesis in this plant (Stonebloom and Scheller, 2019).
Before large-scale cultivation of T. kok-saghyz in a particular district or country, it is essential to know how the season, age, and harvesting time affect natural rubber production in this species. In the case of guayule, it was found that rubber production was cyclical in nature. The lowest rubber accumulation was found during vegetative growth, with maximum accumulation occurring in fall and winter (Schloman Jr. et al., 1986).
In this study, we investigated seasonal variations in rubber accumulation in T. kok-saghyz experimental fields in South Korea to develop large-scale cultivation of Russian dandelion for natural rubber production.
Materials and Methods
Plant material and sampling procedure
Taraxacum kok-saghyz seeds were obtained from the USA and planted in wild field plots at the Korea Research Institute of Bioscience and Biotechnology (KRIBB), Daejeon, South Korea (Bae et al., 2020; Ganesh et al., 2020; Tata et al., 2012). The seeds were sown and grown for one month in the LED growth room and another month in the greenhouse. Two-month-old young seedlings were transplanted into the field. Three months after transplanting, five plants were randomly harvested at three-month intervals from October 2013 to October 2014. Harvested plants were washed in flowing water to remove soil from the roots, and the samples were dried in the shade for a week. The leaves were removed, and the roots were dried in an oven at 65℃ for a week.
Analytical procedures and data analysis
Dried roots were weighed, ground, and stored in plastic bags. Dried root powders were soxhleted for 24 h in 350 mL of cyclohexane in a 500-mL round-bottomed flask. The soxhleted materials were evaporated using a rotary evaporator. The empty glass bottles were weighed, and the rubber layer formed in the round-bottomed flask was scraped and placed in an empty vial. Then, 5 mL cyclohexane was added to the vial, and the dried rubber materials were dissolved overnight. After dissolution, 10 mL acetone was slowly added to obtain a white rubber precipitate (cyclohexane: acetone=1:2). The samples were then centrifuged at 5,000 rpm for 3 min. The supernatants were discarded, and pure rubber, formed as precipitates, was dried. The collected pure rubber were weighed. Finally, the rubber contents of the plants were calculated. The results were reported as mean ± standard deviation values (Choi et al., 2020).
Statistical analysis
Differences between data points were statistically analyzed by an analysis of variance (ANOVA) followed by Duncan’s test. Statistical significance was set at P < 0.05 using SPSS software (version 22).
Results and Discussion
Morphological changes due to seasonal variations
Seasonal variations affect leaf morphology in T. kok-saghyz. The morphological changes in the plants with changes in seasons were recorded, as indicated in Fig. 1. The leaves were short, shiny, and leathery, with a brownish copper tinge during the spring season (Fig. 1A). Moreover, they were completely prostrate and arranged in a rosette formation. As the summer approached, the leaves showed an erect tufted habit. They were also thinner and longer and had an exhausted appearance (Fig. 1B). However, with the increase in temperature during the summer, the leaf size decreased, and the foliage became less erect (Fig. 1C).
As the fall approached, the leaves regained health and strength (Fig. 1D). However, they became prostrate and narrow with a pale green color in winter and started getting stressed (Fig. 1E). During the late winter, most leaves died and disappeared, although some plants retained a much reduced leaf system.
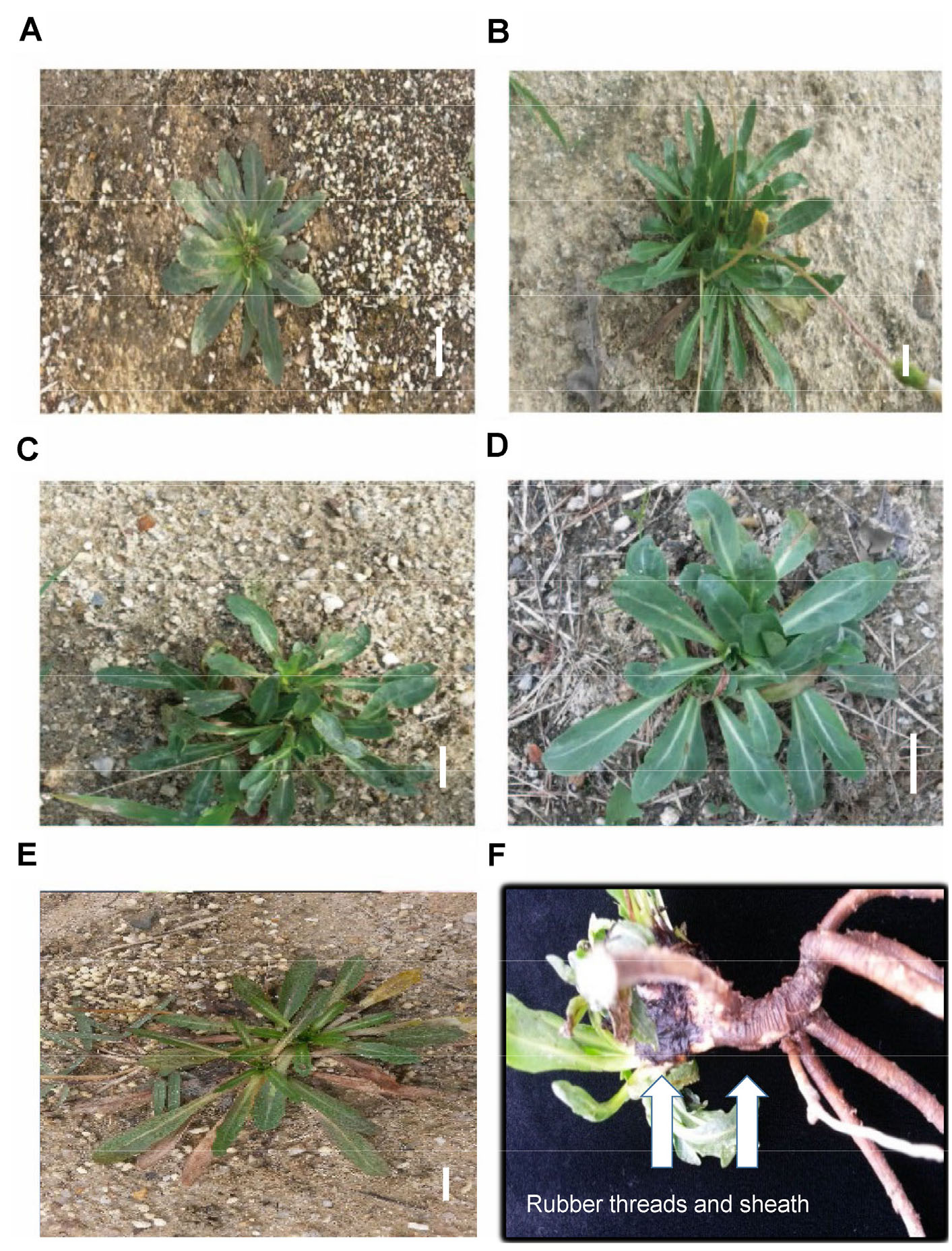
Fig. 1.
Field-grown T. kok-saghyz plant morphology in different seasons, such as early spring (A), late spring-early summer (B), mid-summer (C), late summer-early autumn (D), and late autumn-early winter (E). (1F) shows sloughing off of rubber threads and sheaths that are dark brown to black in color from the root tissues of T. kok-saghyz in summer. Bars = 2 ㎝.
Sloughing-off process in summer-harvested roots
It has been reported that in T. kok-saghyz, solid rubber is sloughed off as threads in the plant tissue and as a rubber sheath around the roots during April and May (Buranov and Elmuradov, 2010) and in the summer (Kreuzerger et al., 2016). Our results match those of previous experiments, as the sloughing-off phenomenon has been observed in the roots of plants harvested in the summer (Fig. 1F). Sloughing off appears as a coagulated lump or brownish sheath in the roots. Although studies have described this phenomenon, the reason for the sloughing-off has not been dealt with in detail until now. However, this phenomenon could explain why the rubber content of the root tissues was lower in the summer than in the spring (Fig. 2A).
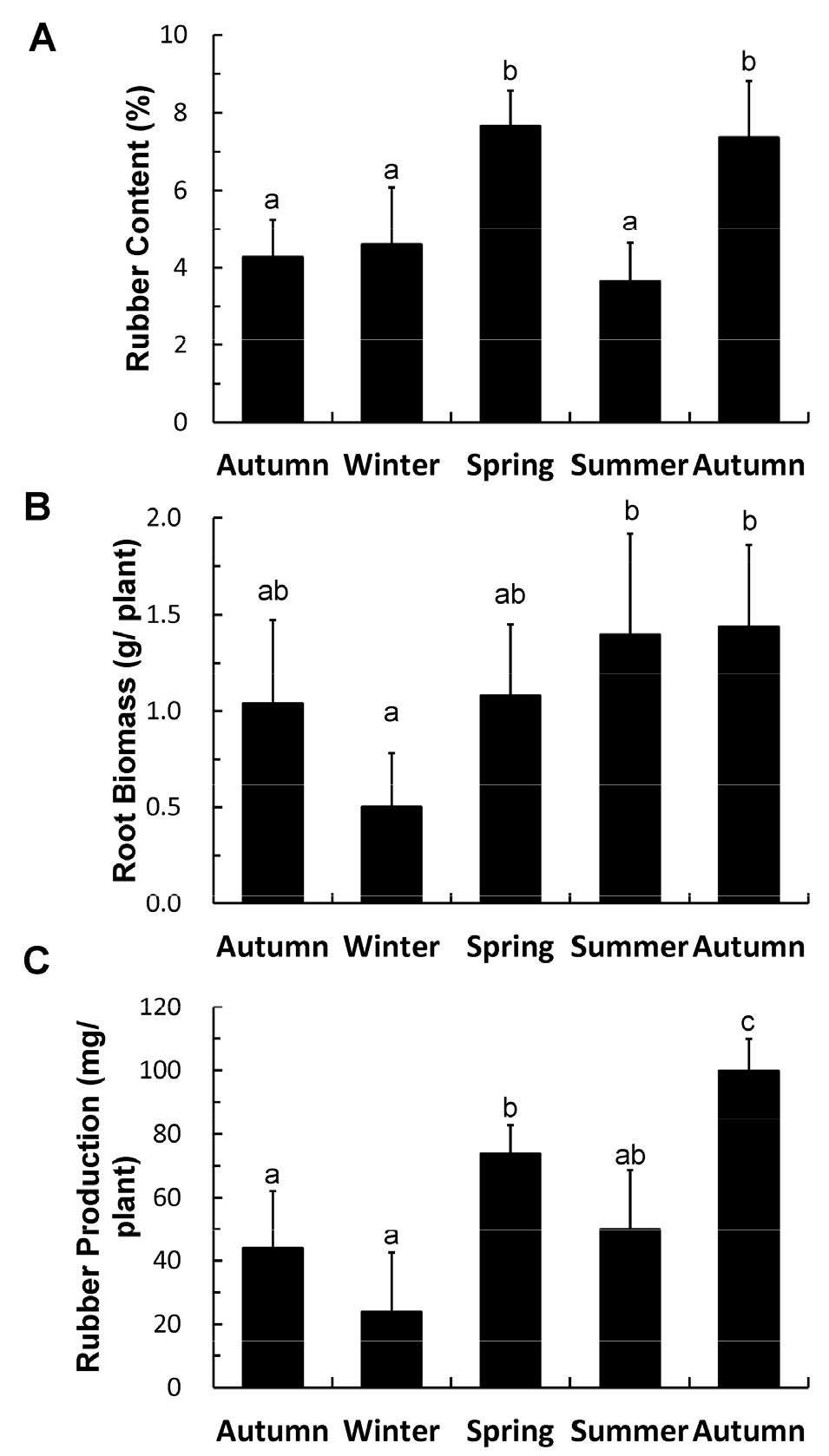
Fig. 2.
Rubber content and root biomass in T. kok-saghyz plants grown in the field in different seasons throughout two years. Rubber content (A) and dry biomass (B) of the root tissues of T. kok-saghyz plants, and rubber production per T. kok-saghyz plant (C). Differences between data points were statistically analyzed by an analysis of variance (ANOVA) followed by Duncan’s test. Statistical significance was set at P < 0.05 using SPSS software (version 22).
Seasonal variation in rubber production of Russian dandelion T. kok-saghyz
Rubber formation in T. kok-saghyz was tracked during different seasons of the year. The T. kok-saghyz rubber content and its variation during different seasons are shown in Fig. 2A. The % rubber contents of the root tissues in autumn and winter were similar, at approximately 4.28% and 4.60%, respectively. With the arrival of the spring, the plants showed an abrupt increase in the rubber content, and the rubber content of the root tissues was found to be approximately 7.66%. The rubber content in the root tissues of T. kok-saghyz plants dropped sharply to 3.65 % in summer but fully recovered to 7.37% in autumn.
In the present study, the effect of seasonal variation on rubber production was studied in T. kok-saghyz. German research has concluded that the best season for the biosynthesis and accumulation of rubber is the spring (Kreuzberger et al., 2016). The increase in the rubber concentration was maximum in the spring (12 months post-field emergence) and the second season. Our group observed a similar increase in rubber percentage during spring (Fig. 2A). We believe that spring supports both the rubber biosynthetic machinery as well as the growth and development of the plants. When the rubber biosynthesis and healthy growth of plants coincide, there is increased rubber production during the spring season. In rubber trees, seasonal variations have been found to affect the enzyme activity related to photosynthesis and sucrose synthesis, which is the precursor of latex biosynthesis (Cairo et al., 2015). This critical observation in the rubber tree suggests similar effects may occur in T. kok-saghyz. When the enzyme activity for sucrose production and hydrolysis increases in a particular season, the latex content and rubber production also increase.
The decline in rubber percentage in the summer could be explained by the sloughing-off phenomenon whereby the root bark loses the rubber in the form of thin strings. Loss of the outer layer of the root with coagulated rubber has been reported in the summer (Kreuzerger et al., 2016). This research group ran two kinds of trial fields (trial-1 and trial-2). Trial-1 plants kept their leaves in the summer. By contrast, in trial-2, the plants underwent a stage of summer resting, leading to the complete loss of leaves and flower heads. Our T. kok-saghyz did not show severe loss of leaves or flowers except for the loss of the outer root layer (Fig. 1C), matching trial-1 in the German study. The German team concluded that the summer resting phenomenon in trial-2 was due to a summer drought. The loss of the outer layer from the roots or the sloughing-off phenomenon appears to be a part of summer dormancy/resting. In Korea, summer resting might not be related to drought since the summer in Korea is a rainy season. Instead, the phenomenon may be related to other environmental conditions, such as hot temperatures and strong sunshine. Summer dormancy has also been considered an intrinsic trait independent of environmental conditions (Voltaire and Norton, 2006).
The percentage and production of rubber in T. kok-saghyz root tissues significantly increased in the second autumn season compared to the first autumn (Fig. 2A), indicating the effects of age. In field-grown plants, however, the rubber content varies with the seasonal changes, and it remains almost constant in some seasons. The percentages of rubber in the first year autumn and next year summer plants were almost the same. This result showed that age was not the dominant or the only factor determining the percentage of rubber in plants. If it were, then the percentage of rubber would continuously increase. However, this was not the case, and the rubber percentage in the autumn and summer plants remained almost the same, with a negligible variation. This result proves that seasonal variation was the reason behind the fluctuation or constancy of the rubber percentage, and it was not solely dependent on age.
The dormancy of the plants in terms of overwintering has been observed (Kreuzerger et al., 2016) and corroborated by our results (Fig. 1 and 2). In the morphology of the winter plants, the leaves became greatly reduced in size and paler in color (Fig. 1E). Moreover, the rubber content per plant and dry root weight per plant were reduced (Fig. 2A and 2B), respectively. This indicates that extremes of temperature during winter induced partial loss of plant root tissues. It should be noted that, despite overwintering, the percentage of rubber in winter-grown T. kok-saghyz was not reduced compared to that in autumn (Fig. 2A). We believe this is because winter chilling does not cause the sloughing-off phenomenon in which the root bark loses rubber in the form of thin strings during summer dormancy. Instead, the overall effect of the freezing and harsh environment during overwintering leads to a reduction in root dry weight per plant without affecting the rubber percentage.
Our study showed that the highest rubber productivity of T. kok-saghyz occurred during spring and autumn (Fig. 2C). Notably, rubber productivity did not continuously increase throughout the year but was up and down season to season. The decreased rubber productivity during winter and summer occurred due to the loss of biomass or low rubber content, respectively. A primary reason for harvesting T. kok-saghyz before summer is that it must be collected before the rubber is lost due to sloughing-off. The rationale is that it is crucial to harvest before the rubber is shed with the old root tissues (Kreuzberger et al., 2016). Moreover, it has been reported that inulin is degraded for re-foliation during spring time and mainly synthesized during the summer when photoassimilates are abundant (Kreuzberger et al., 2016; Stolze et al., 2017). Therefore, harvesting T. kok-saghyz during springtime would save the plant resources for growth and rubber formation instead of allowing them to be diverted to inulin synthesis. However, if both rubber and inulin need to be collected for commercial usage, it would be desirable to harvest T. kok-saghyz just before hot summer (Kreuzberger et al., 2016). Finally, it is necessary to determine the harvesting time by considering the long summer rainy season in Korea. Most T. kok-saghyz plants would be at risk of death under strong sunlight in the oversaturated soil after rain. Therefore, the present study demonstrated that sowing T. kok-saghyz on a large scale in autumn, allowing the plants to grow until spring, and harvesting them just before the arrival of hot and rainy summer, would give the best rubber yield for commercial production of this species in Korea.




