Introduction
Materials and Methods
Plant material
In vitro shoot proliferation
In vitro root induction
Results and Discussion
Effect of high and low auxin treatments in rooting experiment #1
Effect of medium and carbon source types in rooting experiment #2
Evaluation of In vitro root formation in rooting experiment #3
Introduction
Pear (Pyrus spp.) belongs to the Rosaceae family (Potter et al., 2007) and is one of the typical fruit species. It is grown in the temperate climate regions throughout the world (Silva et al., 2014). In general, pear propagation and genetic improvement are performed by conventional breeding. In vitro pear culture was previously reported to improve proliferation rates and plantlet quality by several researchers (Freire et al., 2002; Karimpour et al., 2013; Lucyszyn et al., 2006; Yi et al., 2015). However, despite results on the healthy shoot multiplication, it is still difficult to root pear cultivars in vitro (Thakur et al., 2008). Although this problem has been solved in some cultivars, it remains an essential issue to be solved not only in pear trees but also in most of woody trees (Amghar et al., 2021; Hu and Wang, 1983; López-Pérez and Martínez, 2015).
Developing in vitro regeneration and rooting systems is basically a key tool for plant breeding, cryopreservation, large-scale multiplication, and several aspects of plant development in pear genotypes (Gitta et al., 2019; Yi et al., 2015). Root induction is an important step in the in vitro micropropagation of plant species (González et al., 1991). The in vitro propagation and root-induction of in vitro plants are influenced by different combinations and concentrations of plant hormones (Gitta et al., 2019). Haq et al. (2009) reported that the initiation process of the rooting process was regulated by many factors including culture medium components, but mainly, plant growth regulators (PGR).
The root induction response to auxin has been described as an essential factor for in vitro root induction (Bhojwani et al., 1984). The most effective auxin was reported to be naphthaleneacetic acid (NAA), followed by Indole-3-butyric acid (IBA), indole acetic acid (IAA), and 2,4-dichlorophenoxyacetic acid (2,4-D) (Hu and Wang, 1983). The combination of PGRs, NAA with IBA (Yi et al., 2015), IBA with cytokine (6-benzylaminopurine, BAP) (Gitta et al., 2019), or the addition of activated charcoal (AC) (Shibli et al., 1997) have also been studied. However, those studies reported low rooting rates or the requirement for a the long period of root induction. The development of optimum methods for in vitro root induction is important for rapidly increasing the production rate and plant quality rapidly. This study was conducted to find the most appropriate medium condition for in vitro rhizogenesis of pear accessions using various kinds and concentration of PGR.
Materials and Methods
Plant material
Three pear cultivars (Pyrus L. cv. Bartlett, BaeYun No.3 and Oharabeni) were used as plant material to study improvements in the in vitro root induction system in pear genetic resources. The pear accessions were obtained from the National Institute of Horticultural and Herbal Science (NIHHS) of the Rural Development Administration (RDA). The shoot explants of approximately 1-2 cm in length were used for in vitro shoot proliferation and root induction. All of cultures were maintained in a culture room at 24 ± 1℃ under a 16 h of photoperiod and in closed vessels with stable high humidity.
In vitro shoot proliferation
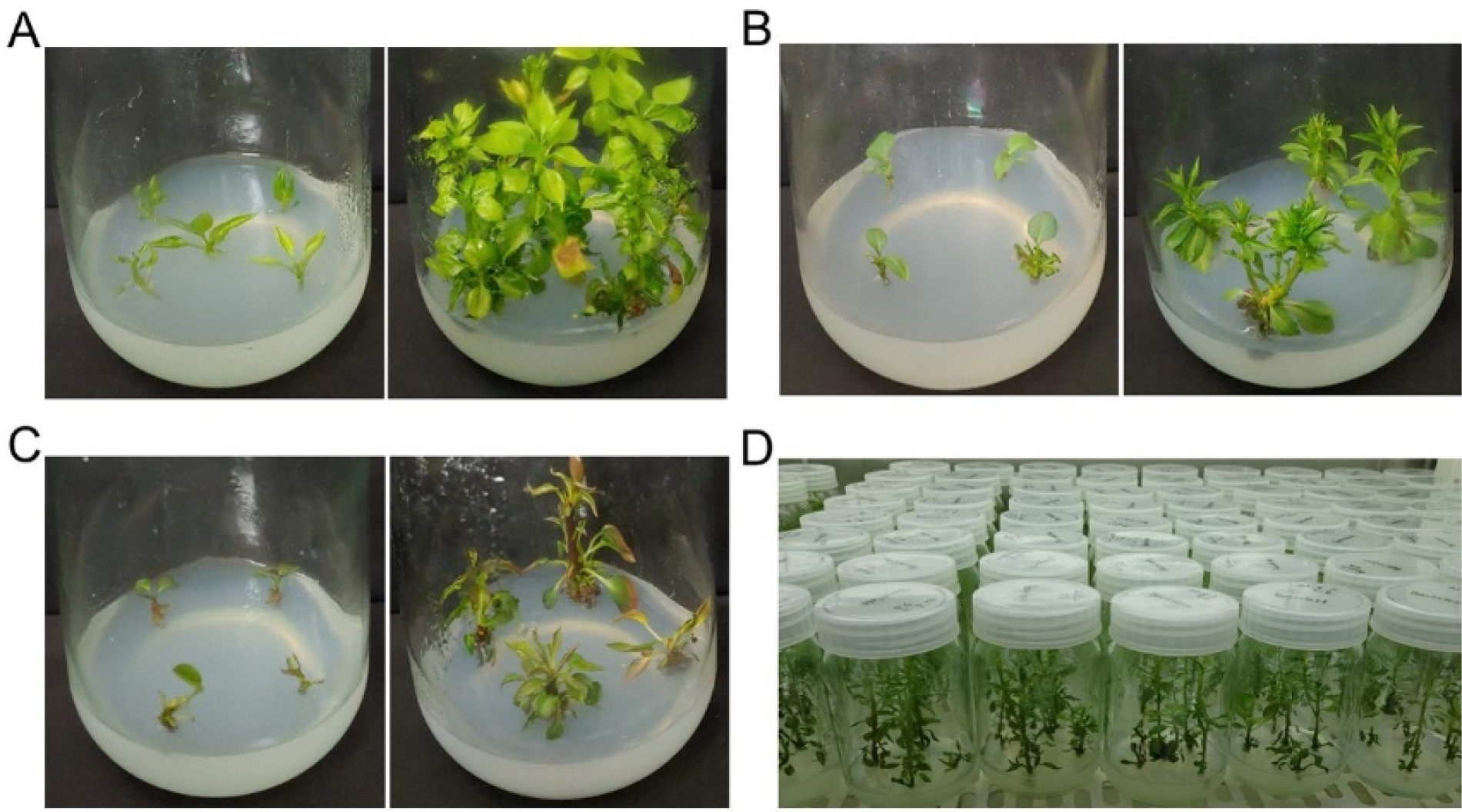
For shoot propagation, the shoot tips and stem explants from three pear genotypes were cultured on Murashige and Skoog (MS) medium (Murashige and Skoog, 1962) with 2.0 ㎎/L N6-benzyladenine (BA) and 0.2 ㎎/L indole-3-butyric acid (IBA) containing 30 g/L sucrose and adjusted pH of 5.8 according to the method previously described by Yi et al. (2015). Well-developed shoots were obtained on the medium (Fig. 1) in 6-week-old cultures and the shoots were used for the in vitro root induction experiments.
In vitro root induction
Rooting experiment #1
The explant of shoots from the propagated shoots of three cultivars, ‘Bartlett’, ‘BaeYun No.3’, and ‘Oharabeni’, were cultured on following media: 1/2 MS containing 0.2 ㎎/L IBA with 15 g/L Sucrose (rooting medium 1, RM1) according to Yi et al. (2015), and 1/4 Linsmaier and Skoog medium (LS) medium containing 1 ㎎/L IBA and 1 ㎎/L NAA with 7.5 g/L glucose (rooting medium 2, RM2) according to Kim et al. (2016), which showed the best rooting response in different pear genotypes. After two weeks, the plants on the RM2 medium were transferred to RM1 medium (RM2to1 condition) (Table 1). The percentage of root induction was investigated after seven weeks.
Table 1.
Composition of media used in this study for in vitro root induction of pear
Rooting experiment #2
To investigate the rooting response of the explants according to the type of medium and carbon source, such as MS or LS and sucrose or glucose, the explants of shoot and stem were transferred to the following four media: 1/2 MS containing 0.2 ㎎/L IBA with 15 g/L sucrose (MS+S) or glucose (MS+G) and 1/2 LS containing 0.2 ㎎/L IBA with 15 g/L sucrose (LS+S) or glucose (LS+G), as shown in Table 1. The root induction frequency in the two cultivars ‘Bartlett’ and ‘BaeYun No.3’ pear was checked after 30 days of cultures.
Rooting experiment #3
Based on the results of experiments 1 and 2, six treatments were used to find the optimal medium for the root induction of explants, as shown in Table 1. In the ‘Oharabeni’ pear genotype, healthy shoots maintained in the propagation medium were transplanted to MS medium without PGR for one week. Nodal explants obtained from the shoots were cultured on the initial root induction medium (R0) in the dark for three days. R0 medium included 1/2 MS with 20 g/L sucrose, 1 ㎎/L IBA, and NAA. The explants on R0 medium were moved to five different root induction media (R1-R5): 1/2 MS with 30 g/L sucrose supplemented PGR-free without (R1) or with 1 g/L charcoal (R2), and 0.2 ㎎/L of IBA and NAA without (R3) or with 1 g/L charcoal (R4), and medium containing 15 g/L sucrose with 0.2 ㎎/L IBA and 1 g/L charcoal (R5). The percentage of root formation, number of roots, and root length were monitored after 14 days of treatments.
Results and Discussion
Torrey (1976) reported that auxins were the main factors related to root formation. Of the auxins, NAA and IBA have been reported to be the most effective factors (Hu and Wang, 1983). Several researchers reported that a correlation between various auxins and root inductions, but the results were low rate and it is difficult in most of pear species (Hu and Wang, 1983; Reed, 1995; Thakur et al., 2008). Therefore, the successful in vitro root induction of this woody species is important (Amghar et al., 2021).
Effect of high and low auxin treatments in rooting experiment #1
For in vitro root induction of the three pear cultivars, ‘Oharabeni’, ‘Bartlett’ and ‘BaeYun No.3’, the shoot and stem explants from the propagated plants were cultured on two different media, RM1 and RM2 media. Our results indicated that callus formed at the explant base on the RM2 medium with high auxins concentrations (NAA and IBA) after two weeks of culture. Similar studies reported that high concentrations of auxin induced callus and inhibited root formation (Lane, 1979), and were undesirable for root development (Thimann, 1977). Hence, the explants on the RM2 medium were moved to RM1 medium (RM2to1 condition). After nearly seven weeks, the results of this experiment #1 showed that percentage of in vitro rooting were 22.2% on RM1 and 30% on RM2to1 conditions in ‘Bartlett’ and 70% on RM1 and 60% on RM2to1 conditions in ‘Oharabeni’ cultivars. No significant differences in the two cultivars were observed between RM1 and RM2to1 conditions. However, ‘BaeYun No.3’ cultivar was observed 0% on RM1 and 72.7% on RM2to1 conditions (Fig. 2 and Fig. 3). The RM2to1 condition yielded higher root induction than that of IBA only. However, in high concentrations of NAA and IBA, the rooting response resulted in callus formation and low-quality roots and required a long period of root induction. Although root induction is inhibited by high concentrations of auxin (Lane, 1979; Thimann, 1977), our results suggest that a brief exposure of explants to high auxin is required for initial root induction and rooting, which is probably the result of increased cell production in the meristem (Beemster and Baskin, 1998).
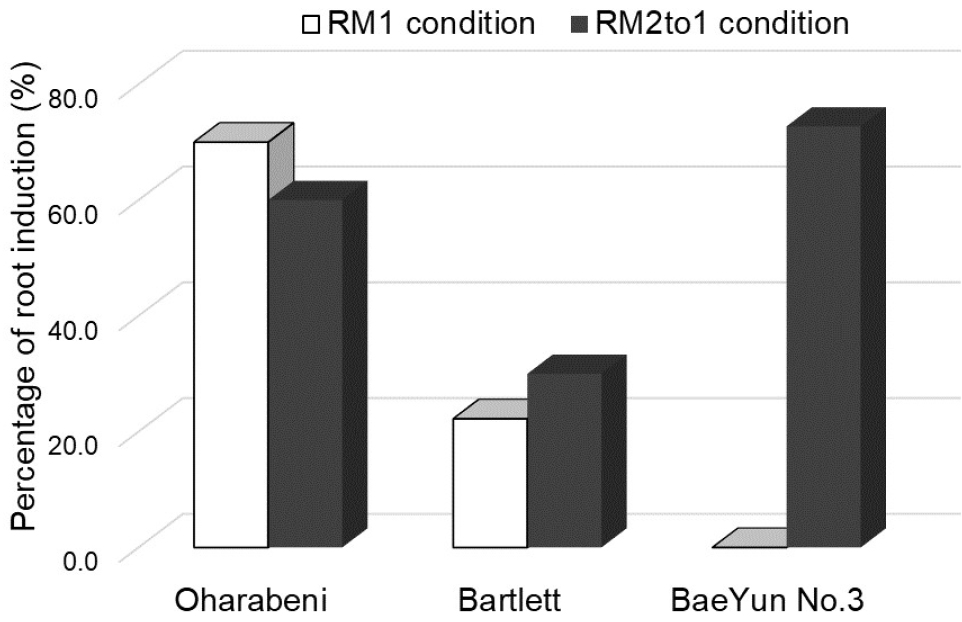
Fig. 2.
Percentage of rooting formation in three pear cultivars after seven weeks. RM1 condition, the plants were cultured on RM1 medium (1/2 MS with 0.2 ㎎/L IBA and 15 g/L Sucrose). RM2to1 condition, the plants were cultured on the RM2 medium (1/4 LS containing 1 ㎎/L IBA and 1 ㎎/L NAA hormone with 7.5 g/L glucose) for two weeks and transferred to RM1 medium.
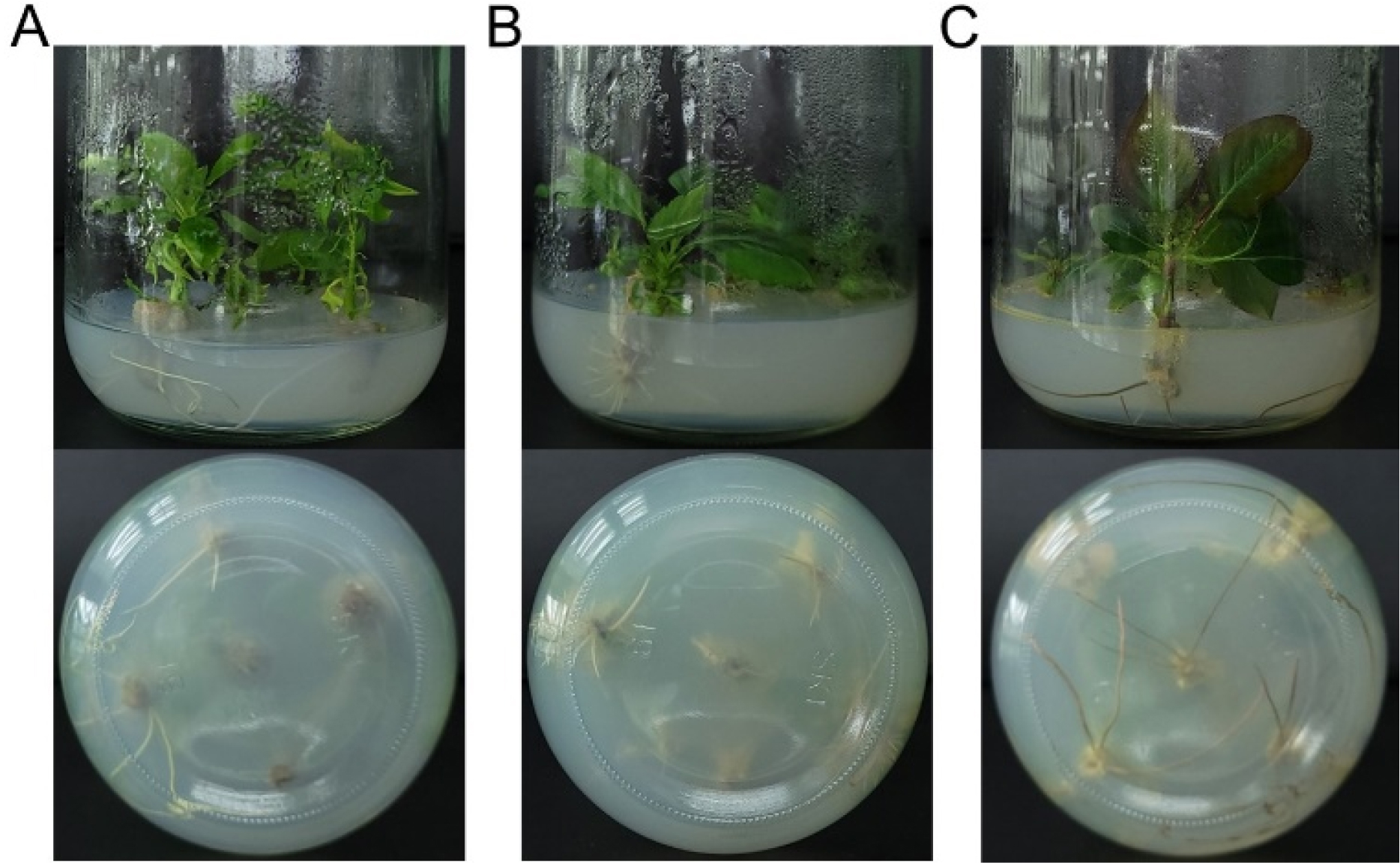
Fig. 3.
In vitro root formation of three pear genotypes. A: Bartlett, B: BaeYun No.3 and C: Oharabeni cultivars formed roots after seven weeks in rooting medium (RM2to1 condition). RM2to1 condition, the plants were cultured on the RM2 medium (1/4 LS containing 1 ㎎/L IBA and 1 ㎎/L NAA hormone with 7.5 g/L glucose) for two weeks and transferred to RM1 medium.
Effect of medium and carbon source types in rooting experiment #2
Previously, Bell and Reed (2002) reported that MS medium is usually used as the mineral medium for many Pyrus species for regeneration and root formation. Sepahvand et al. (2012) reported the number of roots formed was higher in the combination of high concentrations of thiamine and IBA than in the control group. Thiamine is usually provided in the range of 0.1 to 0.4 ㎎/L (Murashige, 1974). The concentration of thiamine is 0.1 ㎎/L in MS medium and 0.4 ㎎/L in LS medium. Hence, we surveyed the effects of different types of medium and carbon sources, such as sucrose and glucose, to determine the optimal rooting medium in two pear cultivars. As shown in Table 2, the root induction rates were 32.5 and 7.5% in MS+S, 30 and 5% in MS+G, 20 and 12.5% in LS+S, and 22.5 and 2.5% in LS+G media for ‘Bartlett’ and ‘BaeYun No.3’ pear cultivars, respectively. The use of MS and sucrose produced good root induction in pear species as compared to LS and glucose. Consequently, MS medium including sucrose was more effective for increasing of root induction from explants in the experiment #2. However, overall, the in vitro rooting rates for 30 days were low, in the range of 2.5 to 32.5% (Table 2).
Table 2.
Effect of medium and carbon source types on root induction of explants in ‘Bartlett’ and ‘BaeYun No.3’ pear after 30 days of culture. Four treatments with 40 explants per treatment
Evaluation of in vitro root formation in rooting experiment #3
The combination of NAA and IBA was more effective for in vitro root formation in pear over NAA or IBA (Thakur et al., 2008). Based on the results of rooting experiments #1 and #2, we tested short pre-treatment in high concentrations (1 ㎎/L) of NAA and IBA (R0 medium) in the dark for three days, followed by transfer to five root induction media (R1 to R5) in the ‘Oharabeni’ cultivar to improve in vitro rooting and reduce the root induction time (Table 3).
Table 3.
Effects of sucrose, charcoal, IBA, and NAA on rooting percentage, number of roots per shoot, and root length after 14 days in the ‘Oharabeni’ pear genotype
|
Rooting media |
1/2 MS (g/L) |
Sucrose (g/L) |
IBA (㎎/L) |
NAA (㎎/L) |
Charcoal (g/L) |
No. of explants tested (ea) |
No. of explants rooted (ea) |
% of rooting formation |
Total no. of roots (ea) |
Mean no. of roots/plant (ea) |
Mean root length (cm) |
| R0z | 2.2 | 20 | 1 | 1 | - | Culture medium for initial root induction | |||||
| R1 | 2.2 | 30 | - | - | - | 12 | 10 | 83.3±8.3 | 31±4.0 | 2.6±2.1 | 3.6±1.9 |
| R2 | 2.2 | 30 | - | - | 1 | 12 | 8 | 66.7±11.8 | 24±3.3 | 2.0±1.6 | 3.9±3.1 |
| R3 | 2.2 | 30 | 0.2 | 0.2 | - | 12 | 10 | 83.3±14.4 | 28±2.6 | 2.3±1.6 | 0.9±0.4 |
| R4 | 2.2 | 30 | 0.2 | 0.2 | 1 | 12 | 8 | 66.7±11.8 | 17±3.4 | 1.4±1.4 | 3.9±3.0 |
| R5 | 2.2 | 15 | 0.2 | - | 1 | 11 | 9 | 81.8±8.3 | 27±0.8 | 2.5±1.6 | 4.2±2.5 |
To confirm the rooting response after culturing on five media, the rooting parameters were evaluated by counting the rooting rate, the total number of roots, the mean number of roots per explants, and the mean root length after 14 days (Table 3). Root formation was observed in all treatments and reached rooting percentages of 66.7% to 83.3% within 14 days (Table 3 and Fig. 4). The highest rooting rate of 83.3% was obtained from explants on R1 and R3 media. R1 medium showed a relatively high total number of roots at 31. Rhizogenesis measured using the rooting parameters was higher when the explants were moved to R1 medium without hormone and charcoal compared to other media. Similar study reported that short exposure of shoots to IBA followed by their transfer to hormone free medium resulted in good rooting response in pear (Marino, 1984). The length and shape of the roots were significantly inhibited in the medium with 0.2 ㎎/L of IBA and NAA without AC (R3 medium), whereas root elongation was induced in the R4 medium with AC (Fig. 4). Thimann (1977) reported that root elongation was inhibited by high concentrations of auxin. However, adding AC to the culture medium decrease the concentration of NAA added to the medium due to the absorption of the growth-regulating substance by AC (Steimitz and Yahel, 1982). Similar results reported that the addition of AC to the medium promoted shoot elongation and suppressed the number of shoots and differentiation (Song et al., 2021; Yang et al., 1992). Our results revealed that the in vitro root formation of explants could readily induce and significantly short the root induction time to 14 days in the ‘Oharabeni’ pear genotype using in vitro rooting system developed in this study (Table 3 and Fig. 4). It was shorter than the 7 weeks in rooting experiment #1, which showed a rooting rate of 60% or more. Liaw et al. (1992) and Yi et al. (2015) reported that root induction took more than 30 days. Also, the induction of roots of pears in vitro has been reported, but the rooting responses were poor (Anirudh and Kanwar, 2008; Bell and Reed, 2002). This method is similar to those reported previously in pear by Marino (1984) which tested a short liquid pre-treatment with IBA.
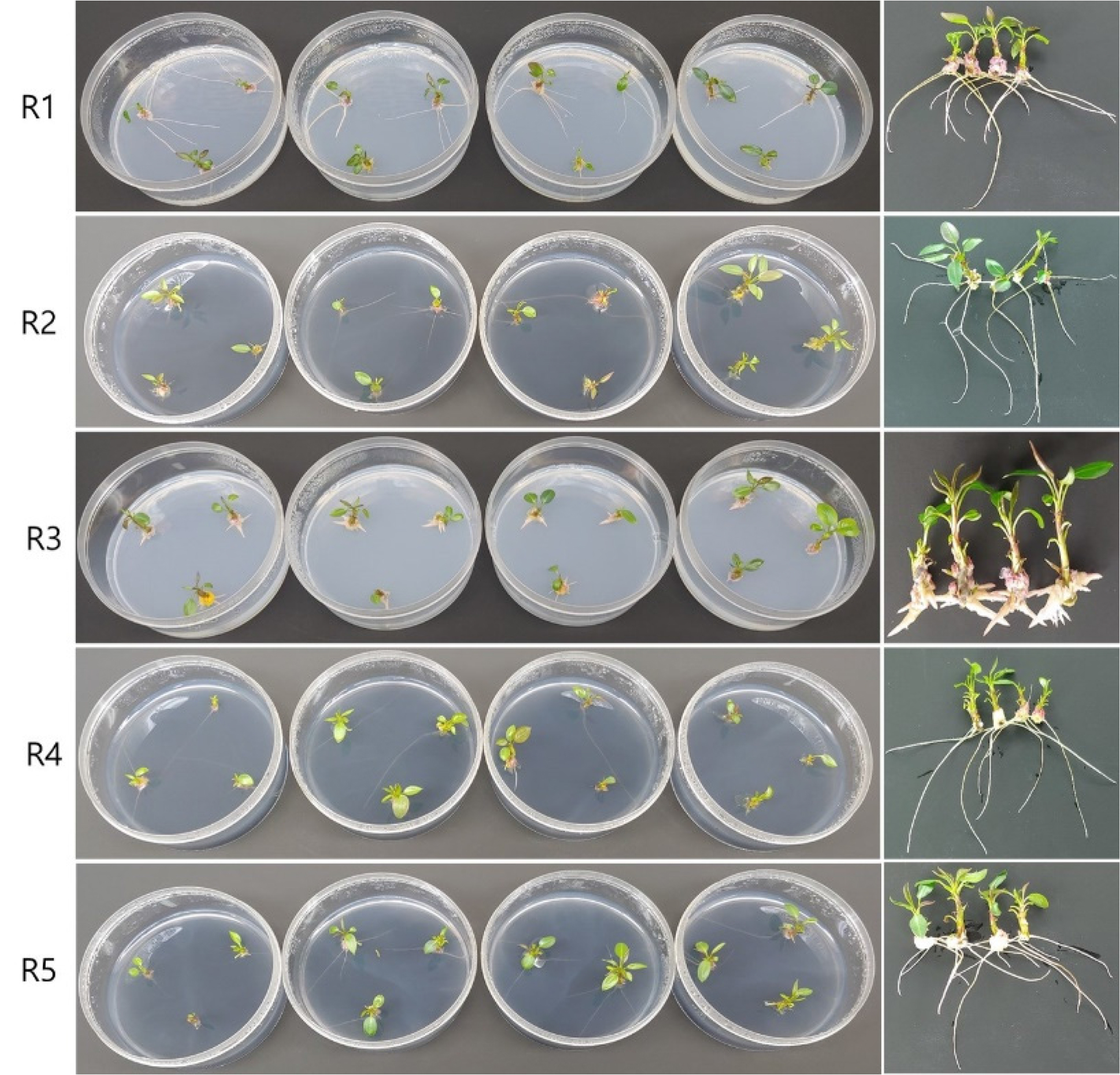
Fig. 4.
In vitro root formation of of ‘Oharabeni’ pear genotype shoot explants after 14 days of treatments. The shoot explants on initial root induction medium (R0) in dark for three days were moved to five media (R1-R5); 1/2 MS with 30 g/L sucrose PGR-free (R1) supplemented with 1 g/L charcoal (R2), and 0.2 ㎎/L of IBA and NAA auxins without (R3) or with 1 g/L charcoal (R4), and containing 15 g/L sucrose, 0.2 ㎎/L IBA, and 1 g/L charcoal (R5).
In conclusion, we described an efficient method for in vitro root formation of pear genotypes. The appropriate protocol for the rooting of explants was pre-treatment on R0 medium (1/2 MS with 20 g/L sucrose, 1 ㎎/L of IBA and NAA) in dark conditions, followed by transfer to R1 medium (1/2 MS with 30 g/L sucrose). These conditions resulted in a good rooting response in a pear genotype. Although a number of protocols have been used for pear species, information on an optimal protocol for in vitro rhizogenesis of various Pyrus species has been lacking. The protocol described in this study could be used for in vitro propagation and root formation of Pyrus germplasm. Further studies to test the suitability of the protocol developed in this study for other pear genotypes are underway.