Introduction
Materials and Methods
Experimental materials
Organic acid analysis
Fermentation solution analysis
Statistical analysis
Results and Discussion
Organic acids content
Sugar content and harvest rate of fermentation solution
Correlation
서언
Plum tree (P. mume) is a deciduous subtree belongs to the Prunus, Rosaceae of China origin, which grows currently wild or cultivated in the warm regions of northeast Asia (Song, 1998). Plum tree in Korean peninsula was planted for processing in the Koryo period following the ornamental of the Three Kingdoms period, and now trees with older age and historical properties are being cultivated rarely.
Plum tree fruit (plum) has a number of bioactive substances, exhibiting antibacterial activity, appetite improvement, sterilization, insecticidal action with the prevention of various adult diseases (Yun, 2011).
In addition, plums are mainly used for processing plum wine, drink, concentrate, vinegar, pickle, tea (Lim and Eun, 2012; Yaegaki et al., 2006), so researches on food properties and efficacy have been variously conducted. The main studies of plum are useful components (Cha et al., 1999b; Kim et al., 2006), and pharmacological effects (Kim et al., 2002; Seo et al., 2008), organic acids (Cha et al., 1999a; Paik et al., 2010) and plum fermentation solution (Seon et al., 2017; Yun, 2011).
Although most of these studies were conducted on improved varieties, resource characteristics of the historical old plum were extremely rare. In particular, the usefulness study for the plum resources in the distribution of all regions in Korea is expected to provide lots of information about the availability of plum.
This study was analyzed on the characteristics of organic acids and plum fermentation solution for the plum fruit of 192 germplasms in order to provide useful information of the plum trees use collected in all regions of South Korea.
Materials and Methods
Experimental materials
The study was conducted on 192 germplasms of P. mume (plum trees) planted on Kongju university farm (Fig. 1). Plum trees are collected and grafted in South Korea and then grown for more than 10 years. Identification of the plum tree was utilized in the literature of Lee (2003). Experimental materials are plum tree fruits (plums) harvested from 2018 to 2019. Organic acid contents of plums were analyzed by selecting 43 germplasm according to the distribution ratio of fruit flesh color and weight.
Organic acid analysis
The plum samples were collected in June 2018 and stored at -70℃ up to 3 months prior to experimental use. After thawing, plum seeds were removed and remaining fleshes were cut into small pieces. One gram of sample was placed in a 50 mL centrifuge tube, and 30 mL of 20 mM potassium phosphate were added and homogenized with a polytron (PT 2500 E, Kinematica AG, Switzerland) at a speed of 1,000 rpm. After centrifugation (Combi-514 R, Hanil, Republic of Korea) at 3,000 x g for 10 min, supernatants were filtered through a syringe filter (nylon, 0.45 ㎛, ADVANTEC, Japan) and injected into an HPLC system (S2100, Sykam, Germany). Table 1 shows HPLC instrumental conditions for organic acid analysis and resultant chromatograms for a plum sample and authentic standards of oxalic (OA), malic (MA), and citric (CA) acids are provided in Fig. 2A. Under our experimental conditions OA, MA, and CA showed retention times of 2.92 min, 3.87 min, and 5.75 min, respectively (Fig. 2A). Authentic standards for OA, MA, and CA were purchased from Sigma-Aldrich (USA) and standard mixture solutions ranging 10 to 250 ppm, 150 to 3,750 ppm, and 400 to 10,000 ppm, respectively, were prepared to evaluate plum germplasm samples with diverse range of organic acid contents. Validations for organic acid analysis methods were performed prior to sample analyses. The recovery rates for OA, MA, and CA tested at 3 different levels with 6 replications for each level were 91.3%-98.9%, 90.8%-97.6%, and 84.0-89.1%, respectively. Limit of detection (LOD) and limit of quantitation (LOQ) were 2.88 and 8.74 ppm for OA, 36.8 and 111.5 ppm for MA, while CA showed 81.4 and 246.7 ppm, respectively. Relative standard deviations (RSD, in %) for repeatability tests based upon 6 replicated injections of 7 levels of authentic standards and reproducibility tests based upon 6 inter-date injections of 7 levels of standards were 1.1-3.2% and 1.5-4.8% for OA, 1.0-2.9% and 2.0-4.7% for MA, and 1.0-3.4% and 1.9-4.3% for CA, respectively.
Table 1.
Operation conditions of HPLC for organic acid analysis
| Item | Condition |
| Column | Zorbax SB-Aq (4.6 ㎜ × 250 ㎜) |
| Mobile phase | 20 mM potassium phosphate |
| Flow rate | 1.0 mL‧min-1 |
| UV detector | 220 ㎚ |
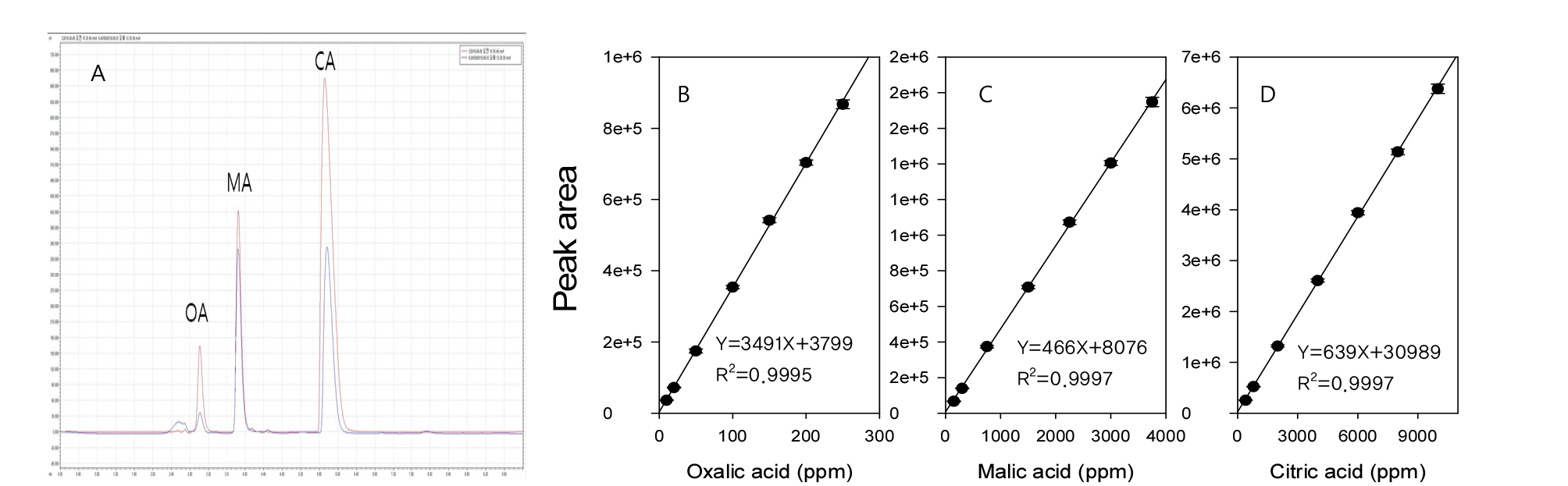
Fig. 2.
Chromatograms for sample (blue) and authentic standards (red) (A) and their calibration curves for oxalic (B), malic (C), and citric (D) acids. The retention times for OA, MA, and CA were 2.92 min, 3.87 min, and 5.75 min, respectively. Data in calibration curves represent mean values ± standard deviation of 6 independent replications.
Fermentation solution analysis
The plums of experimental materials were used through the process of washing and drying after harvesting in June 2019. The plums of 200 to 300 g were stored at normal temperature by sealing up in a plastic airtight container with the same amount of white sugar. Sugar content of fermentation solution was measured three times by opening a closed container in October 2019 with a fruit sugar index meter (Giwon high- tech GMK-706R). Sugar content was analyzed by selecting a uniform 148 germplasms of fermentation solution, the total harvest rate of 192 germplasms is shown in fermentation solution (mL)/weight (g) of plum.
Statistical analysis
Statistics analyzed the correlation and average difference between characteristics using the SPSS 23.
Results and Discussion
Organic acids content
The content of organic acids by fruit flesh color is the same as Table 2. The total organic acid content was 50.9 ± 6.0 ㎎/g. In the contents by fruit flesh colors, orange color was the highest as the 54.3 ± 8.5 ㎎/g, the next, light orange (52.3 ± 4.2 ㎎/g) and whitish green (48.1 ± 4.7 ㎎/g) was followed. In the contents by organic acids, citric acid was the highest as the 28.3 ± 9.0 ㎎/g, 55.5%, the next, malic acid (22.1 ± 5.2 ㎎/g, 43.4%) and oxalic acid (0.55 ± 0.32 ㎎/g, 1.1%) was followed. It was similar to the report of So (2013) that the citric acid was contained the highest as 2.0∼3.3 ㎎/mL in the plums.
Table 2.
Comparison of organic acid contents by fruit flesh colors of P. mume germplasms
yMean separation within columns by Duncan's multiple range test at p = 0.05.
Content of oxalic acid and malic acid was a few in difference by fruit flesh color, but the citric acid content showed a difference, which the orange color was the highest and whitish green was the lowest. The organic acids content in fruit flesh color was different; whitish green did show little difference in the content of malic acid and citric acid, but citric acid is higher 20.0%, 18.6%, respectively than the malic acid in the light orange and orange.
The content of organic acids by plum weight is the same as Table 3. The content of malic acid, citric acid with total organic acid did not show a difference by weight, and oxalic acid showed a difference by weight. In oxalic acid, the weight 5.1∼10.0 g (0.77 ± 0.27 ㎎/g) was the highest, and the more than 20.1 g (0.21 ± 0.24 ㎎/g) was the lowest.
Table 3.
Comparison of organic acid contents by fruit weight of P. mume germplasms
ynot significant.
In more than 20.1 g weight, the difference between citric acid and malic acid is very little, but other weight groups were 5.7 to 27.4% higher than citric acid.
In citric acid content, the lower plum weight grew, the higher tendency was. The citric acid content contained the most commonly in the plum showed a certain tendency in other studies; malic acid and oxalic acid content is reduced by plum maturation and showed annual variations, but citric acid was increased by plum maturation and no showed annual variations (Gwak et al., 2018; Kim, 2017).
Sugar content and harvest rate of fermentation solution
The sugar content of the fermentation solution was an average of 55.7 ± 1.6 °Brix, range 48.8 to 59.7 °Brix (Table 4). Sugar content distribution was the highest in 54.1 to 56.0 °Brix (37.0%), followed by 56.1 to 58.0 °Brix (29.2%), 52.1 to 54.0 °Brix (12.5%).
Table 4.
Distribution of sugar content (°Brix) of fermentation solution of P. mume germplasms
| D\ivision | Sugar content (°Brix) | Range | Mean ± S.D | C.V (%) | |||||
| 50.0 below | 50.1 ~ 52.0 | 52.1 ~ 54.0 | 54.1 ~ 56.0 | 56.1 ~ 58.0 | 58.1over | ||||
| rate (%) | 1.6 | 6.5 | 12.5 | 37.0 | 29.2 | 2.6 | 48.8 ~ 59.7 | 55.7 ± 1.6 | 2.9 |
The sugar content of the fermentation solution showed a variety of individuals, in spite of the same ratio and the same fermentation period of the plum and sugar, but did not show a significant difference in the individuals because variation coefficient is lowered to 2.9. Therefore, the sugar content of the fermentation solution is believed to affect more difference of the sugar ratio and fermentation period than the same conditions. So (2013) reported that the longer the fermentation period became, the less sugar of the plum fermentation solution grew.
The harvest rate of the fermentation broth was wide as an average of 116.7 ± 8.7%, range 91.3 to 134.1%, as table 5, the coefficient of variation was higher than the sugar content indicating 7.4. The distribution of yield sped up from 110.1 to 115.0% to 23.4%, followed by 120.1 to 125.0% (20.8%), 115.1 to 120.0% (20.3%) it was followed by:
Table 5.
Distribution of the harvest rate of fermentation solution of P. mume germplasms
| Division | Harvest rate (%)z | Range | Mean ± S.D | C.V (%) | |||||
| 105.0 below | 105.1 ~ 110.0 | 110.1 ~ 115.0 | 115.1 ~ 120.0 | 120.1 ~ 125.0 | 125.1 over | ||||
| rate (%) | 9.3 | 9.9 | 23.4 | 20.3 | 20.8 | 16.2 | 91.3 ~ 134.1 | 116.7 ± 8.7 | 7.4 |
Correlation
Correlation coefficients between sugar content and weight of the plum, correlation coefficients between the sugar content of fermentation solution and harvest rate were very low. The relationship harvest rate between the fermentation solution sugar content and weight of the plum was the correlation coefficient of r=0.180*, r=0.150*, respectively, they marked the relevance between the two characteristics because showed significance by 5% (Table 6).
Table 6.
Correlation coefficients for soluble solid content (°Brix), fruit weight, the sugar content (°Brix) and harvest rate of fermentation solution of P. mume germplasms
| Division | Fruit weight | Sugar content (°Brix) | Harvest rate |
| Soluble solid content (°Brix) | 0.055 | 0.125 | -0.052 |
| Fruit weight | - | -0.086 | 0.150* |
| Sugar content (°Brix) | - | - | 0.180* |
The relationship between the plum weight and the organic acid content had a tendency of lowing the content of oxalic acid when the plum weight was heavier, because the oxalic acid and plum weight was recognized as r=-0.551** correlation. Correlation by organic acid showed a correlation of r=-0.767** between the malic acid and citric acid, and r=0.834** between citric acid and the total organic acid (Table 7).
Table 7.
Correlation coefficients for fruit weight and organic acid contents of P. mume germplasms
| Division | Oxalic acid | Malic acid | Citric acid | Total organic acid |
| Fruit weight | -0.551** | 0.270 | -0.203 | -0.103 |
| Oxalic acid | - | 0.039 | -0.142 | -0.126 |
| Malic acid | - | - | -0.767** | -0.288 |
| Citric acid | - | - | - | 0.834** |
In the above results, the main organic acid of the plum was citric acid, malic acid, oxalic acid as reported by Kim (2017). The total organic acid content of the plums was lots of changes in high and low contents by the individual and organic acids, and the ratio of the content was also different by the individual. The diversity of this organic acid content is inferred to be related to the taste and aroma and harvest rate of the plum fermentation solution, there it needs the relevance of these elements by various analytical methods in the future.
In the fermentation solution of the plum, because of variety of individuals in sugar content and harvest rate, the possibility of developing a variety of products utilizing these resources will be greatly increased, if the additional researches are performed on this project.





