Introduction
Materials and Methods
Plant Materials and Seed Culturing
Callus Induction and Protocorm like Bodies Formation
Pretreatment of Different Culture Temperature
Statistical Analysis
Results
Callus Induction and PLBs Formation from Seed Culturing
Effect of Suspension Culture
Effect of Different pH on the Properties of Coconut Water
Effect of Different Pretreatment Temperatures
Discussion
Introduction
Orchids are one of the most pampered plants and occupied the top position among all flowering plants for cut-flower production and potted ornamentals (Parab and Krishnan, 2012). Orchids are important in the floriculture industry due to their beautiful foliage, colorful and fragrant flowers of varying shapes, and the long vase life of cut flowers. They also have numerous medicinal properties (Bhattacharjee et al., 2015). Many species of Orchidaceae grow in the world, consisting of 35,000 species and 800 genera (Singh et al., 2007). Many commercially important orchids are now grown artificially for their high value both in domestic and international markets (Kumar et al., 2002).
Bulbophyllum is one of the largest genera in the Orchidaceae family. This genus comprises about 3,000 species (Siegerist, 2001). Because of a limited distribution area and specific habitat, this species is at risk of extinction in its natural habitat. In vitro propagation techniques have been widely used for conservation and have succeeded in many orchid species, including Dendrobium (Da Silva et al., 2015; Hossain et al., 2013; Parthibhan et al., 2015; Zhao et al., 2013) and Cymbidium species (Da Silva and Tanaka, 2009; Mohanty et al., 2012; Teixeira da Silva, 2012). In vitro culture and micropropagation of Bulbophyllum species have been reported in previous studies (Bhadra and Hossain, 2004; Lee and Yeung, 2010; Maneerattanarungroj et al., 2010; Than, 2013) and factors for improving shoot multiplication in Bulbophyllum species, especially medium components were also studied (Myo et al., 2012; Pal et al., 2009; Vij et al., 2000). Normally, Bulbophyllum auricomum Lindl. can be propagated by asexual methods using pseudobulbs. In nature, seed propagation cannot be found because seeds do not have sufficient nutrients to support the growth of embryos to full plants. Orchid seeds are minute, non-endospermic, and have a reduced embryo enclosed within a more or less transparent coat. Thus, the rate of germination in nature is very low. Apart from the difficulties of seed germination, the life cycle of orchids is very long in natural conditions. It takes from five to ten years for a plant to bloom and produce fertile seeds. Vegetative propagation of orchids is also an extremely slow process. Therefore, mass scale production of orchids depends heavily on different in vitro methods. Such methods could be effectively used for conservation purposes in both direct and indirect ways (Mondal and Banerjee, 2017).
Previously, in vitro propagation of B. auricomum Lindl. was performed with MS (Murashige and Skoog, 1962) medium supplemented with various plant growth regulators (PGRs) and coconut water collected from explant parts (Naing and Lim, 2011; Than, 2013). Therefore, the objective of this study was to determine the effects of the addition of coconut water to the MS basal medium with or without plant growth regulators. Moreover, we investigated the effects of pH and temperature in suspension culture for the in vitro propagation of B. auricomum Lindl.
Materials and Methods
Plant Materials and Seed Culturing
The flowers of B. auricomum Lindl. bloom with inflorescence in late fall to early winter. Full blooms of this flower were used for artificial pollination. Following the artificial pollination, mature pods were obtained after four months. Fully grown pods were selected for in vitro seed culture. The pods were rinsed with running tap water for 20 min and soaked in 25% (w/v) sodium hypochlorite (NaClO) for 15 min. The pods were then sterilized with 75% ethanol for 3 min and soaked three times in sterilized distilled water to remove chemical residue. After that, the following processes were carried out on a clean bench. The pods were longitudinally divided by a surgical blade and inoculated aseptically on MS basal medium supplemented with 3% sucrose, and 0.8% agar (pH was adjusted to 5.6±1 before autoclaving). Culture media were incubated in a culture room at 25±2℃ under long day condition (16 h light/ 8 h dark cycle) with a cool-fluorescent white light intensity of 3000 lux.
Callus Induction and Protocorm like Bodies Formation
In vitro propagation of B. auricomum Lindl. was carried to the following treatment.
(i) After three months of seed germi-ation culture, callus was observed on MS basal medium.
(ii) To obtain a callus suspension culture, calli (0.23 g of fresh weight) were sub-cultured on MS liquid medium supplemented with three different concentrations (0.5, 1.0, and 3.0 ㎎/L) of PGRs, such as 2,4-dichlorophenoxy acetic acid (2,4-D) with (2g/L) activated charcoal (AC) by shaking in an incubator for one week. After one week of suspension culture, calli were taken by filtering from different concentrations of 2,4-D liquid medium and sub-cultivated on three different media compositions of MS basal medium supplemented with 150 mL/L of coconut water (CW), 2.0 ㎎/L of BAP (6-benzyl aminopurine) and MS (control).
(iii) Then, MS basal medium supplemented with 150 mL/L CW in six different values of pH (4.8, 5.0. 5.2, 5.4, 5.6, and 5.8) was used for the formation of protocorm-like bodies (PLBs).
Pretreatment of Different Culture Temperature
Two-month-old in vitro raised PLBs were grown as small pseudobulb plantlets. To establish temperature variation, explants sized in 7-8 ㎝ were cultured on the MS basal medium at 15℃ in a refrigerator, 25℃ in a culture room, and 35℃ in an incubator for one month pretreatment. During these culture processes, the explants were sub-cultured on new MS medium containing two different combinations and concentrations of natural extracts such as coconut water and PGRs such as MCBN: MS medium supplemented with 150 mL/L CW along with 2.0 ㎎/L BAP and 1.0 ㎎/L NAA (1-napathylacetic acid); MBN: MS medium in combination with 2.0 ㎎/L BAP and 1.0 ㎎/L NAA. For each culture temperature, an experimental process was conducted during four successive weeks. After that, these culture media were kept at 25±2℃.
All media were supplemented with 3% sucrose, 0.8% agar, and 0.2% activated charcoal (AC) for solidified medium. The media were adjusted to pH 5.6±1 and sterilized with an autoclave at 121℃ (1.5 kg/㎠ pressure) for 15 min. Culture media were incubated in a culture room under cool fluorescent tubes for 16/8 h (light/dark) photoperiods at about 3000 flux light intensity and relative humidity of 25±2℃. Soon after preparation, about 30 mL of the media were poured into 250 mL sized culture boxes and each experiment involved 10 bottles.
Statistical Analysis
The experiments were performed three times with three replicates each time (n = 3). Data are expressed as means±standard deviation. The mean values were analyzed statistically by Tukey’s test (one-way ANOVA). All the statistical analyses were carried out through GraphPad Prism (9.3.0).
Results
Callus Induction and PLBs Formation from Seed Culturing
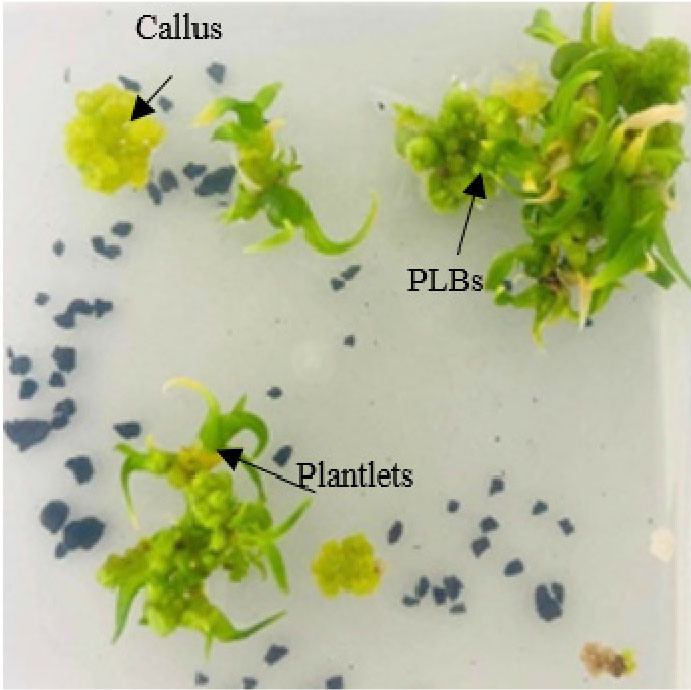
Seed germination after culturing were recorded when most viable seeds have been germinated. MS basal medium was successful in seed germination by 90% (i.e., seed germination was formed on 9 out of 10 boxes) and the development of protocorm formation to plantlet regeneration and callus induction of B. auricomum Lindl. after culturing for 1.5 months (Fig. 1).
Effect of Suspension Culture
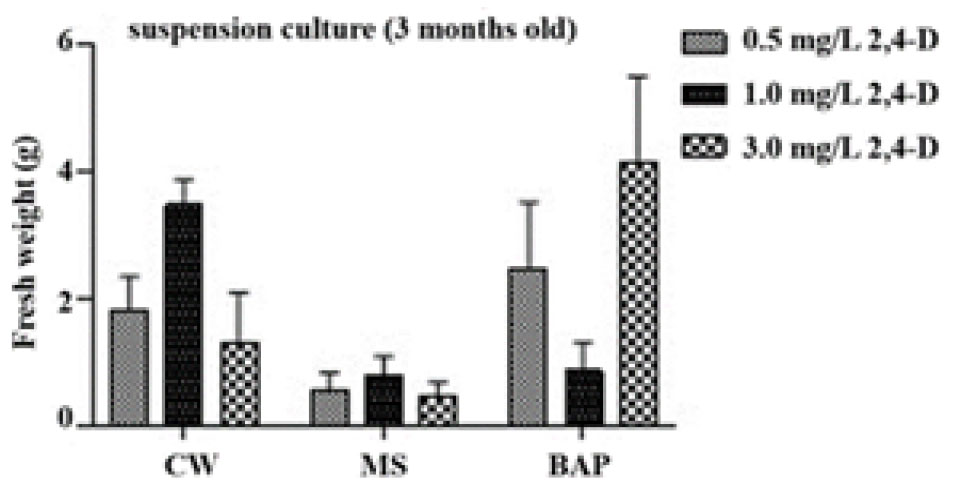
In the results of two months of culture, the fresh weights of the induced callus and formed PLBs of each culture medium were measured. The best callus induction (4.1±1.4) was observed on MS medium supplemented with 2.0 ㎎/L BAP from a suspension culture of MS basal medium supplemented with 3.0 ㎎/L 2,4-D and 2 g/L AC, followed (3.5±0.4) by MS medium supplemented with 150 mL/L CW from the suspension culture of MS medium supplemented with 1.0 ㎎/L 2,4-D, and the least (0.5±0.2) was observed on MS (control) from the suspension culture of MS medium in combination with 3.0 ㎎/L 2,4-D (Figs. 2 and 3). However, MS medium supplemented with 150 mL/L CW showed better PLBs formation compared with MS medium containing 2 ㎎/L BAP. Therefore, the coconut water medium was chosen for next experiments.
Effect of Different pH on the Properties of Coconut Water
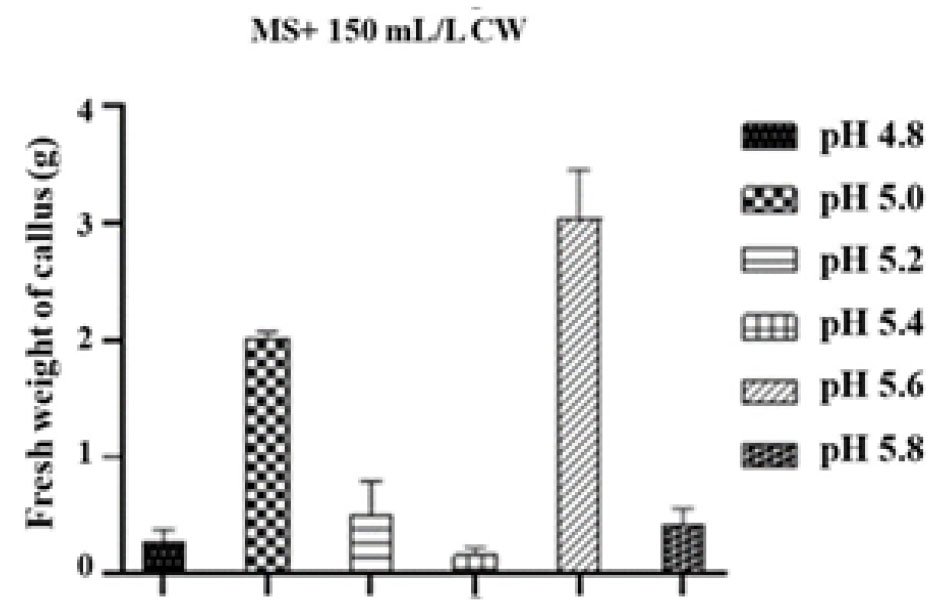
Regarding the impact of pH on the performance of coconut water, pH from 4.8 to 5.8 were used. According to the results, a significant proliferation of PLBs was observed on MS medium supplemented with 150 mL/L CW at pH 5.0 and 5.6. Remarkably, the medium with pH 5.6 showed a higher formation of PLBs (3.0±0.4) than that with pH 5.0 (2.0±0.1) after three months (Figs. 4 and 5).
Effect of Different Pretreatment Temperatures
Small pseudobulb plantlets derived from PLBs were sub-cultured on MS basal medium and pretreated at different temperatures, setting to 15℃, 25℃, and 35℃ for one month. Then, they were transplanted into two new media: MCBN and MBN. Results showed that MCBN produced a maximum number of plantlets (2.7±0.6) on 15℃ at the earlier stage of the second week compared to MBN (Table 1). MBN produced the highest number of leaves culture (2.3±0.6) at 25℃ at the second week culture compared to MCBN (Table 2). Similarly, MBN favored the length of leaves (4.7±0.2) on 15℃ at three weeks (Table 3). Combining the results of Fig. 6, Table 1, 2, and 3, there was no significant difference in the number of plantlets and leaves in the MCBN and MBN media. But, it was confirmed that MBN is more effective than MCBN on the length of leaves (p < 0.05).
Table 1.
A comparative effect of temperature and culture media on a number of plantlets of B. auricomum Lindl.
zMCBN: MS basal medium supplemented with 150 mL/L coconut water along with 2 ㎎/L BAP (6-benzyl aminopurine) and 1 ㎎/L NAA (1-napathyl acetic acid); MBA: MS medium in combination with 2 ㎎/L BAP and 1 ㎎/L NAA. ySuperscript letters (a, b, c, and d) in each row indicate statistically significant differences at p < 0.05 (Tukey’s test). *No survival in new culture medium; **Died in the incubator at 35℃; ***All data obtained from three replicates represent mean±SD.
Table 2.
Effect of temperature and culture media on the number of leaves of B. auricomum Lindl.
Footnotes are same as Table 1.
Table 3.
Analysis of the effect of temperature and culture media on the length of leaves of B.auricomum Lindl.
Footnotes are same as Table 1.

Fig. 6.
Effect of pretreatment at 15℃ and 25℃ on the development of shoot formation of B. auricomum Lindl. after 3 months of culture. (a) Number of plantlets formed at 15℃ at the second week on MCBN; (b) Number of leaves observed at 25℃ from the second week; (c) Length of leaves was high at 15℃ at the third week on MBN, respectively.
Discussion
This study demonstrated a high effect of MS basal medium on the seed germination of B. auricomum Lindl. Similar results were also reported by Than in her previous study (Than, 2013). And also, Popova et al. (2003), reported that MS basal medium containing nitrogen and ammonium stimulates seed germination (Popova et al., 2003). An 2,4-D auxin was supplemented as a hormone in the suspension culture, and the majority of authors used 2,4-D at various concentrations to establish cell suspension culture (Soomro and Memon, 2007) and 2,4-D was effective in organ differentiation of in vitro culture of Angelica gigas N. (Lee et al., 2012). In addition, callus induction on MS with 2.0 ㎎/L BAP treatments using nodules plants has been studied (Swamy and Sinniah, 2016). Van Overbeek et al. (1941) stated that coconut water was able to induce early PLBs formation (Van Overbeek et al., 1941). It was demonstrated that MS medium in combination with 150 mL/L CW from MS medium supplemented with 1.0 ㎎/L 2,4-D of suspension culture greatly affected callus induction and PLBs formation. This is in line with previous researchers who reported similar results (Van Overbeek et al., 1941). Scholten and Pierik reported that the pH of the medium has an effect on the availability of many minerals (Scholten and Pierik, 1998). According to the result of this work, the greatest amount of callus induction and PLBs formation was obtained with a pH of 5.6. It is critical to adjust the pH of the culture medium to ensure that the physiological processes of the explants are not disrupted. About the effect of temperature, there was an increase in the number of plantlets at 15℃ on MCBN compared to MBN. But the difference was not statistically significant. The results are shown in Table 1. As demonstrated in Table 2, 25℃ is a better temperature for increasing the number of leaves in MBN than MCBN medium. But there was no significant difference. In the Table 3 we have indicated that 15℃ had a better effect on the length of leaves in MBN than MCBN medium and the difference was statistically significant (p < 0.05). The acclimatization of in vitro under low temperatures (15-25℃) gave the best results for the production of high levels of photosynthetic pigments and enhanced the growth of Phalaenopsis orchid plantlets (Cha-um et al., 2010). In epiphytic orchids, the addition of 15% coconut water stimulated growth in new shoots (Paris et al., 2019) and previous researchers also reported a successful shoot regeneration from protocorms using coconut water (Jainol and Jualang, 2015; Jawan et al., 2007). Our results are in agreement with their reports.
In conclusion, our study revealed that four month old mature pods from artificial self-pollination has to be identified for in vitro seed germination of B. auricomum Lindl. orchid on MS basal medium. Furthermore, MS basal medium supplemented with 150 mL/L CW from suspension culture of MS medium containing 1.0 ㎎/L 2,4–D should be prioritized for callus induction and PLBs formation of B. auricomum Lindl. as demonstrated in this study. The pH of 5.6 has a better effect on the the callus and PLBs formation. Regarding the effect of temperature, MCBN showed the highest number of plantlets from two weeks at 15℃. The same 15℃ at the third week favored the leaves length on MBN medium. Our overall findings demonstrated that in vitro propagation of B. auricomum Lindl. can be helpful to conserve this endangered orchid plant. However, proper selection of the pH and temperature should be considered.