Introduction
Materials and Methods
Results and Discussion
Taxonomic description and distribution characteristics
Specimens observed
Key compared with morphologically similar species of related Korean genera (Korean name) (Fig. 3)
Harmfulness and characteristics of weeds
Weed risk assessment
Taxonomic description and distribution characteristics
Specimens observed
Key compared with morphologically similar species of related Korean genera (Korean name) (Fig. 5)
Harmfulness and characteristics of weeds
Weed risk assessment
Taxonomic description and distribution characteristics
Specimens observed
Key compared with morphologically similar species of related Korean genera (Korean name) (Fig. 7)
Harmfulness and characteristics of weeds
Weed risk assessment
Taxonomic description and distribution characteristics
Specimens observed
Key compared with morphologically similar species of related Korean genera (Korean name) (Fig. 9)
Harmfulness and characteristics of weeds
Weed risk assessment
Taxonomic description and distribution characteristics
Specimens observed
Key compared with morphologically similar species of related Korean genera (Korean name) (Fig. 11)
Harmfulness and characteristics of weeds
Weed risk assessment
Taxonomic description and distribution characteristics
Specimens observed
Key compared with morphologically similar species of related Korean genera (Korean name) (Fig. 13)
Harmfulness and characteristics of weeds
Weed risk assessment
Taxonomic description and distribution characteristics
Specimens observed
Key compared with morphologically similar species of related Korean genera (Korean name) (Fig. 15)
Harmfulness and characteristics of weeds
Weed risk assessment
Taxonomic description and distribution characteristics
Specimens observed
Key compared with morphologically similar species of related Korean genera (Korean name) (Fig. 17)
Harmfulness and characteristics of weeds
Weed risk assessment
Taxonomic description and distribution characteristics
Specimens observed
Key compared with morphologically similar species of related Korean genera (Korean name) (Fig. 19)
Harmfulness and characteristics of weeds
Weed risk assessment
Taxonomic description and distribution characteristics
Specimens observed
Key compared with morphologically similar species of related Korean genera (Korean name) (Fig. 21)
Harmfulness and characteristics of weeds
Weed risk assessment
Taxonomic description and distribution characteristics
Specimens observed
Key compared with morphologically similar species of related Korean genera (Korean name) (Fig. 23)
Harmfulness and characteristics of weeds
Weed risk assessment
Introduction
The features of species on the earth are the product of evolutionary adaptations that have survived over a long period. More than 350,000 species of plants that occupied the land ecosystem for the first time are growing in diverse ecosystems around the world, and about 4,000 species of native plants are currently distributed in Korea (Korea National Arboretum, 2021b). All countries of the world concentrate enormous efforts on securing and preserving plant genetic resources, which are fundamental materials for developing national competitiveness. The Convention on Biological Diversity (CBD) and the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES) are increasingly interested in recognizing genetic resource status and conservation (Convention on Biological Diversity, 2014).
In this study, the term ‘Invasive Alien Species (IAS)’ is defined as the species, which is designated by the Korean government, that has entered and spread into ecosystems and threaten or adversely affect ecosystems linked to biodiversity (Yoon et al., 2019). The IAS emerged as a menacing factor affecting biodiversity and ecological system since the 20th century (IUCN, 2010; Park et al., 2015). Currently, the settlement, reproduction, and diffusion of alien species continue to increase for the following reasons: 1) growth in global commerce due to the development of efficient transportation methods, 2) rapid increase in intercontinental and international movement caused by the increased income levels, 3) disturbance and destruction of ecosystems due to human interference, and 4) climate change (Hobbs and Mooney, 2005). In the United States and Europe, management is carried out in consideration of the inflow and spread of IAS, and international conventions such as CBD are discussing the management of IAS under climate change as the main issue.
The number of exotic plants in Korea is increasing rapidly; 110 species of exotic species in 1980 (Yim and Jeon, 1981), 181 species in 1995 (Park, 1995), 292 species in 2003, 321 species in 2011 (Lee et al., 2011), and 342 species in 2021 (Korea National Arboretum, 2021a). Meanwhile, the Ministry of Environment designated organisms that damage the ecosystem among the introduced species since 1998 as ‘Ecosystem Disturbing Species’, and 16 out of 34 species were reported as plants (Ministry of Environment, 2020). These invasive plants occur in the entire region of South Korea, from Jeju Island to DMZ (Koh et al., 2006; NamGung et al., 2019), therefore, research on pre-control measures is essential to counter these species.
Since putative invasive alien plants have not flowed into the Korean peninsula, native plants have no tolerance for resistance, defense, and competitiveness to non-invasive species. Therefore, based on the aspect, characteristics, and current situation of agricultural environmental damage of IAS already flowing in, it is necessary to take measures to remove putative invasive alien plants in the early stage. Above all, it is most important to limit the influx of non-infiltrated plants into Korea through exhaustive quarantine management of the Animal and Plant Quarantine Agency.
In order to efficient block of putative invasive alien plants, we conduct an agricultural environmental risk assessment of 41 species designated as potential harmful plants by the Ministry of Environment in 2016 (Ministry of Environment, 2016) based on an evaluation sheet (Animal and Plant Quarantine Agency, 2016). Also, this research was conducted to provide detailed data such as taxonomic information including morphological characteristics and classification key for putative invasive alien plants (Hyun et al., 2020; Yoon et al., 2019). We aim to provide useful information on putative invasive alien species by reporting data on 11 species of such plants.
Materials and Methods
We identify un-introduced environmentally harmful plants of the Korea peninsula by investigating means such as specimens, literature, and other information including seeds morphology/classification key, etc. To carry out an agricultural environmental risk assessment (Ministry of Environment, 2016) of these species, we implement the field survey four times: 1) in the western region of the USA including California, Washington, and Oregon from 1st to 8th, August 2017, 2) eastern USA including Texas, Florida, Georgia, South Carolina, North Carolina, Virginia, and Maryland from 8th to 17th of July, 2018, 3) Sydney and Melbourne of Australia from 15th to 23rd of February, 2019, and 4) Mexico and Chicago of the United States from 26th, July to 4th, August, in 2019. By way of habitat survey to figure out the characteristics of distribution concerning putative alien plants, we conducted taxonomic reviews and habitat circumstances survey in detail.
Eleven of the 41 putative invasive species surveyed are presented along with descriptions of their natural habitat and taxonomic key with diagnostic characters : Spirodela punctata (G.Mey.) C.H.Thomps. (Araceae), Sagittaria graminea Michx. (Alismataceae), Elodea nuttallii (Planch.) H.St. John, Hydrocharis morsus-ranae L., Stratiotes aloides L. (Hydrocharitaceae), Eichhornia azurea (Swartz) Kunth, Monochoria hastata (L.) Solms (Pontederiaceae), Aegilops tauschii Coss. (Poaceae), Myriophyllum heterophyllum Michx. (Haloragaceae), Bunias orientalis L. (Brassicaceae), Heracleum sosnowskyi Manden. (Apiaceae) (Fig. 1, Table 1).
Table 1.
Investigated plants list in this study in accordance with APG Ⅳ System
We prepared the photograph of investigated species and habitat. Also, dried specimens from overseas countries including United States, Sweden, and Australia were observed.
Results and Discussion
The following information of 11 species are written based on description provided by Kim (2020).
Spirodela punctata (G.Mey.) C.H.Thomps., Rep. (Annual) Missouri Bot. Gard. 9: 28 (1898)
Korean Name : Jeom-gae-gu-ri-bap (점개구리밥; Ministry of Environment, 2019).
Common Name : Dotted duckweed, Dotted duckmeat, Giant duckweed.
Taxonomic description and distribution characteristics
Taxonomic description: Tiny free-floating aquatic plants. Roots (1-)2-7(-12), all perforating scale. Fronds 1 to 10, narrow ovate, obovate, elliptical or reniform, 1.5-8 ㎜ long, 1.5-3 ㎜ wide, 1.5 to 2 times longer than its width, light green (a waxy layer composed cuticles sparkles in the sunlight), underside occasionally red; veins 3 to 7. Vegetative propagation (occasionally sexually, by seed); daughter fronds is connected to the mother by a thin white stipe. Flowers tiny saccate, ovaries 1-2-ovulate, stamens 2. Fruits with 1 seed (or 2), laterally winged to apex. Seeds with 10-15 distinct ribs. Chromosome number 2n=40, 46, 50 (Flora of China Editorial Committee, 1994; Flora of North America Editorial Committee, 1982).
Growing conditions: This species grows well in shallow ponds and ditches.
Origin: Australia, western Asia. Some reported that its native is North America (BONAP, 1999; USDA-NRCS, 2020), but there are no evidence (Landolt, 1986).
Invaded areas: This species is now widespread throughout diverse continents (such as Asia, Africa, South and North America).
Current state of designations: In USA, this species was listed in noxious plant list (USDA-NRCS, 2020).
Specimens observed
[CANB] 533866.1 (B.J. Lepschi & T.R. Lally BJL 2543, 1996. 3. 10. Australia); 789864.1 (R.W. Purdie, 2010. 4. 24. Australia); 810488.1 (I. Crawford, 2008. 3. 13. Australia); 910218.1 (R.W. Purdie, 2019. 3. 18. Australia); 910227.1 (R.W. Purdie, 2019. 2. 17. Australia); 910229.1 (R.W. Purdie, 2019. 2. 17. Australia) [CONN] 00087351 (Robert S. Capers, 2009. 11. 26. United States); 00154967 (John E. Ebinger, 1971. 8. 16. United States); 00154968 (Gordon C. Tucker, 2005. 10. 14. United States) [TEX] George Yatskievych 18-254, 2018. 10. 3. United States (Fig. 2).
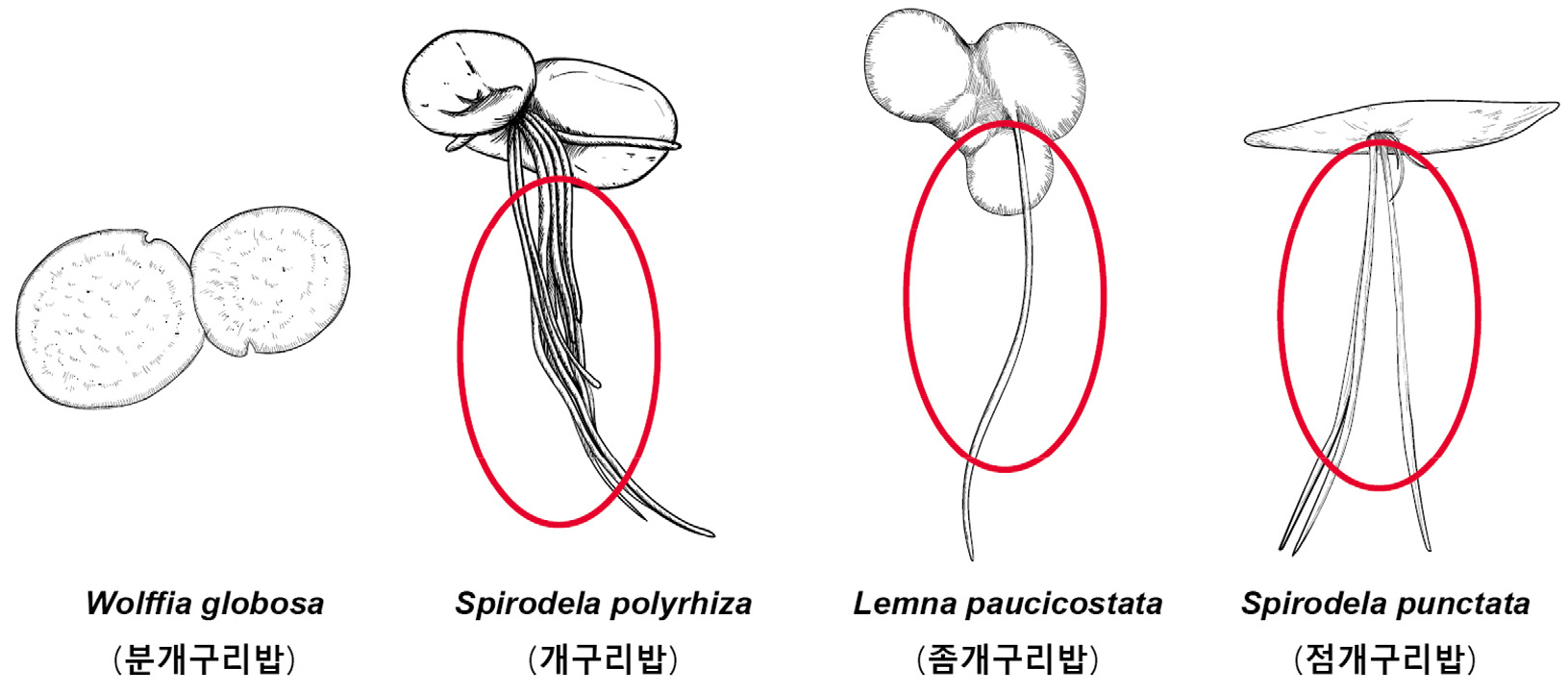
Key compared with morphologically similar species of related Korean genera (Korean name) (Fig. 3)
1. Roots absent and veins in the thallus Wolffia globosa (분개구리밥)
1. Roots present (1-21) and veins (1-21) in the thallus 2
2. Thallus with only one root and the base covered with nothing Lemna paucicostata (좀개구리밥)
2. Thallus with 2 to 21 roots and the base covered with scale 3
3. Thallus with 7 to 21 roots and one of them penetrates membranous scale Spirodela polyrhiza (개구리밥)
3. Thallus with 2 to 7 roots and all roots penetrate membranous scale Spirodela punctata (점개구리밥)
Harmfulness and characteristics of weeds
S. punctata is found to be toxic and causes mutations in higher plants and algae. It can increase the toxicity of oncogenesis, nervous system, heart disease, and pathogens, and has a negative impact on biodiversity. As a result, it is verified that this species is harmful to aquatic organisms and humans. It was known to have been distributed outside of its origin in the 1800s but was mainly found near the harbor, so it is presumed that it was introduced by humans across the continent (Landolt, 1986). This species is widely utilized as an aquarium plant, and it is thought that it spread in the process of transporting fish or plants (Daubs, 1962; Landolt, 1986). If this plant comes into new regions, it can easily spread for a few kilometers depending on the animal. But unlike other floating aquatic plants, this species has the property of drying up in 30 minutes to 2 hours and 30 minutes once water is dried or disappeared (Landolt, 1986). In Jeju Island of Korea, there are several records of invasion. Therefore, it is necessary to focus on the invasive potential of this species (Choi et al., 2018; Kim et al., 2017).
Weed risk assessment
By the result of weed risk assessment criteria (Animal and Plant Quarantine Agency, 2016), the degree of risk is 43% (Table 2). We suggest that this species could be treated as an ordinary weed.
Sagittaria graminea Michx., Fl. Bor.-Amer. (Michaux) 2: 190 (1803)
Korean Name : Jan-di-ip-so-gwi-na-mul (잔디잎소귀나물; Ministry of Environment, 2019).
Common Name : Grassy arrowhead, Grass-leaved arrowhead, Chinese arrowhead, Weatherby’s arrowhead.
Taxonomic description and distribution characteristics
Taxonomic description: Herbs, to 100 ㎝. Aquatic perennial, having rhizomes. Leaves are submerged or out of the water. Submerged leaves are phyllodial, angled abaxially, flattened adaxially, 6.4-35 ㎝ long and 0.5-4 ㎝ wide. Emergent leaves have triangular petiole, 6.5-17 ㎝ long. Blade 2.5-17.4 ㎝ long and 0.2-4 ㎝ wide. Inflorescence racemes or panicles of 1-12 whorled flowers, emersed, 2.5-21 ㎝ long and 1-8 ㎝ wide; peduncles 6.5-29.7 ㎝ long. From sepals not enclosed flowers to 2.3 ㎝ in diameter; pubescent dilated filaments are shorter than anthers; pistillate flowers pedicellate, absence of ring of sterile stamens. Fruits 0.6-1.5 ㎝ in diameter; achenes oblanceoloid, 1.5-2.8 ㎝ long and 1.1-1.5 ㎜ wide, beaked; soft abaxial wings 0-1, glands 1-2 (Smith, 1895) (Fig. 4). Chromosome number 2n=22 (Harada, 1956).
Growing conditions: This species grows well in aquatic environments such as marshes, rivers, and lakes.
Origin: Eastern North America (Missouri Botanical Garden, 2020).
Invaded areas: Great Britain, Trinidad-Tobago, China, Vietnam, Washington (POWO, 2019).
Current state of designations: It is introduced mainly as an ornamental aquatic plant and becomes a serious harmful plant once it flows in. Accordingly, this species is in the Washington quarantine list and its transport, sale and purchase are prohibited (WAC 16-752-610, 2021). Also, its class weed is B in Washington (PNW-IPC, 1998).
Specimens observed
[TEX] George Yatskievych 18-183, 2018. 7. 15. United States.
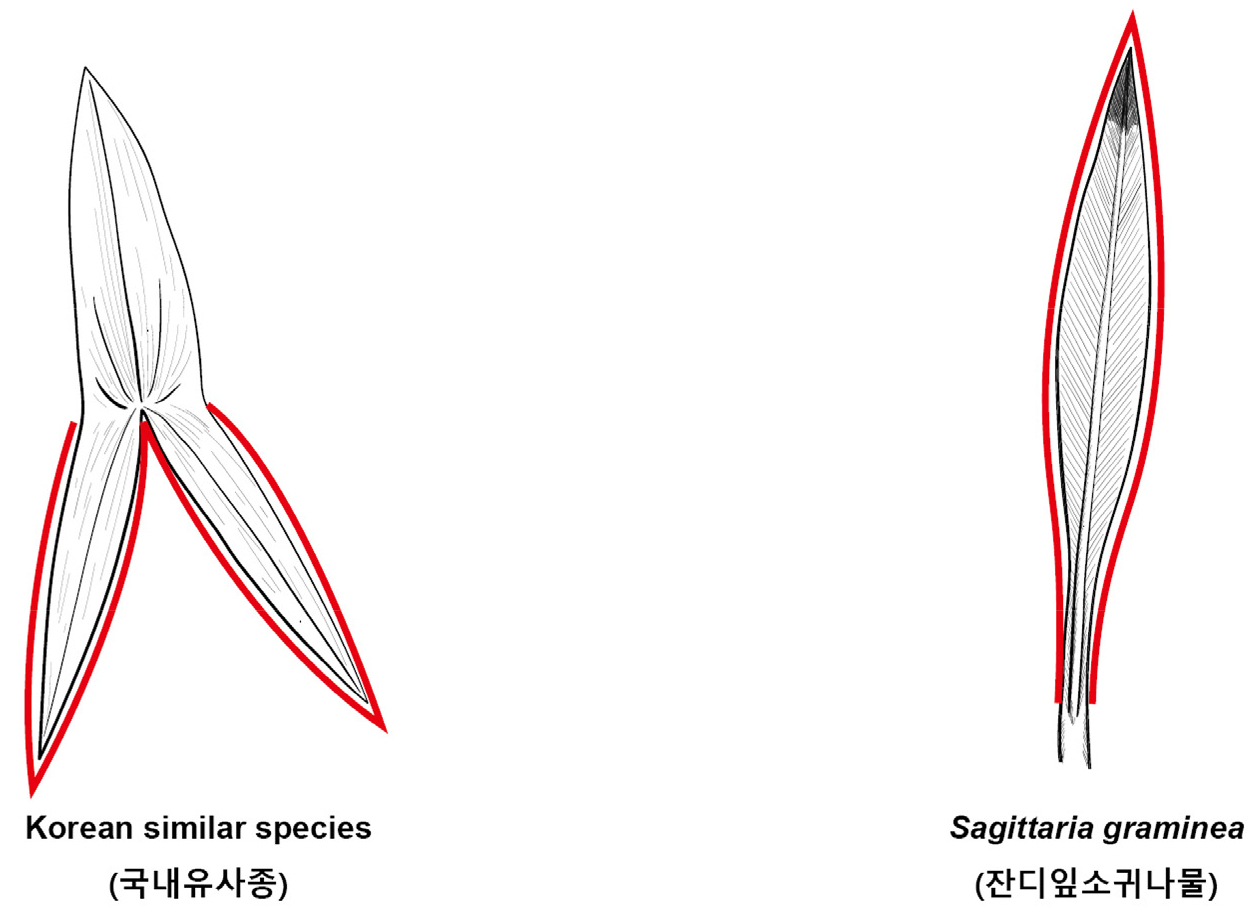
Key compared with morphologically similar species of related Korean genera (Korean name) (Fig. 5)
1. Petioles absent Sagittaria pygmaea (올미)
1. Petioles present 2
2. Leaves with petiole lanceolate, ovate, linear at the top; lower parts are extended 3
3. Stolon present, leave apex lanceolate, ovate; shorter than lower parts 4
4. Corms 2~3 ㎝ diam.; wild Sagittaria trifolia (벗풀)
4. Corms 5~10 ㎝ diam.; cultivate Sagittaria trifolia subsp. leucopetala (소귀나물)
3. Stolon absent, leave apex lanceolate, linear; longer than lower parts Sagittaria aginashi (보풀)
2. Parts of leaf with petiole not extended; leaves linear, oblanceolate Sagittaria graminea (잔디잎소귀나물)
Harmfulness and characteristics of weeds
This species is introduced mainly as ornamental aquatic plant. Due to varied reproductive means, this plants spread out in many parts of freshwater environments rapidly. It threatens native aquatic plants and animal populations. Increased habitat density can limit water flow and affects biodiversity (Zhang et al., 2018). It can affect the irrigation system by reducing water flow. It affects recreational activities such as fishing, swimming and boating, and reduces the visual comfort of waterways (Hamel and Parsons, 2001). It is distributed in the area of average temperature of 4-27℃, and it is possible to invade all parts of the Korean peninsula.
Weed risk assessment
By the result of weed risk assessment criteria (Animal and Plant Quarantine Agency, 2016), the degree of risk is 54% (Table 2). We suggest that this species needs to be registered to control temporarily in law and to investigate the potential risk.
Elodea nuttallii (Planch.) H.St.John, Rhodora 22: 29 (1920)
Korean Name : Kae-na-da-mal (캐나다말; Ministry of Environment, 2019).
Common Name : Nuttall’s waterweed, Free-flowered waterweed, Nuttall’s pondweed, slender waterweed, Western waterweed, Western elodea, Waterweed western.
Taxonomic description and distribution characteristics
Taxonomic description: Perennial submerged-root aquatic plant, dioecious with floating flowers. Roots white, unbranched, at nodes. Stems long and slender, circular section, freely branched, 30-100 ㎝ long. Leaves pale green, regularly arrange in the middle of stem; 3 whorled upper leaves with folded edge, linear or lanceolate, 3-15.5 ㎜ long and 0.9-1.7 (up to 2.4) ㎜ wide; bottom leaves in pair, small, ovate-lanceolate. Dioecious with only one floating flowers (rarely monoecious), within 8 ㎜ in diam.; petals 3, white, waxy. Staminate flower spathes round or ovate, 2.2-4 ㎜ long; sepals ovate, 2 ㎜ long, sometimes red; petals absent or 0.5 ㎜ long and ovate-lanceolate; stamens 9, inner 3 filaments fused basal aspect, anther 1-1.4 ㎜ long, pollen in tetrads. Pistillate flower spathes at leaf axil, linear, 8.5-15 ㎜ long; little sepals green, obovate, 1 ㎜ long; petals white, obovate, longer than sepals; styles 1-2 ㎜ long. Capsules with several seeds, narrow ovate to fusiform, 5-7 ㎜ long; seeds with trichome at base, scrobiculate vertically, fusiform, 3.5-4.6 ㎜ long (Flora of North America Editorial Committee, 1982; Larson, 1993). Chromosome number 2n=32, 42, 44, 48, 56; high level of polymorphism (Flora of North America Editorial Committee, 1982).
Growing conditions: This species occurs mainly in the placid waters of the lake, reservoir, pond, river and so on (Fig. 6).
Origin: North America.
Invaded areas: Europe, China South-Central, Japan (POWO, 2019).
Current state of designations: This species is introduced as an aquarium plant to Europe (the first Belgium) (Hussner, 2012; Preston and Croft, 1997; Simpson, 1984) and later to Japan (Kunii, 1984), but it is generally recognized as a weed in these areas. The European and Mediterranean Plant Protection Organization (EPPO) includes this species on its list of invasive plants (EPPO, 2021). Belgium and Switzerland also backlisted this species because they decide its environmental hazards are high. On the other hand, this plant is not recognized as a weed in its native habitat and is listed on the threatened plant list in Kentucky and Tennessee in the USA (USDA-NRCS, 2020).
Specimens observed
[CM] 362301 (Allison W. Cusick, 1991. 11. 7. United States); 362384 (Allison W. Cusick, 1991. 9. 12. United States); 467336 (M. Loree Speedy & Mark Bowers, 2008. 8. 10. United States); 468285 (Steven P. Grund, 2005. 8. 18. United States); 514086 (Andrew M. Hoff, 2001. 7. 28. Unites States); 524622 (Bonnie L. Isaac, Joseph A. Isaac & Paul A. Isaac, 2009. 9. 5. United States) [CONN] 00062214 (N. Murray, 2006. 6. 26. United States); 00063860 (Robert S. Capers, 2005. 8. 4. United States); 00064078 (Robert S. Capers, 2005. 7. 8. United States) [LD] 1522269 (Håkan Wittzell, 2011. 8. 6. Sweden); 1771785 (Kjell Eriksson & Ulla Eriksson, 2013. 9. 4. Sweden); 1873108 (?, 2002. 8. 4. Sweden) [TEX] George Yatskievych 18-179, 2018. 7. 15. United States.
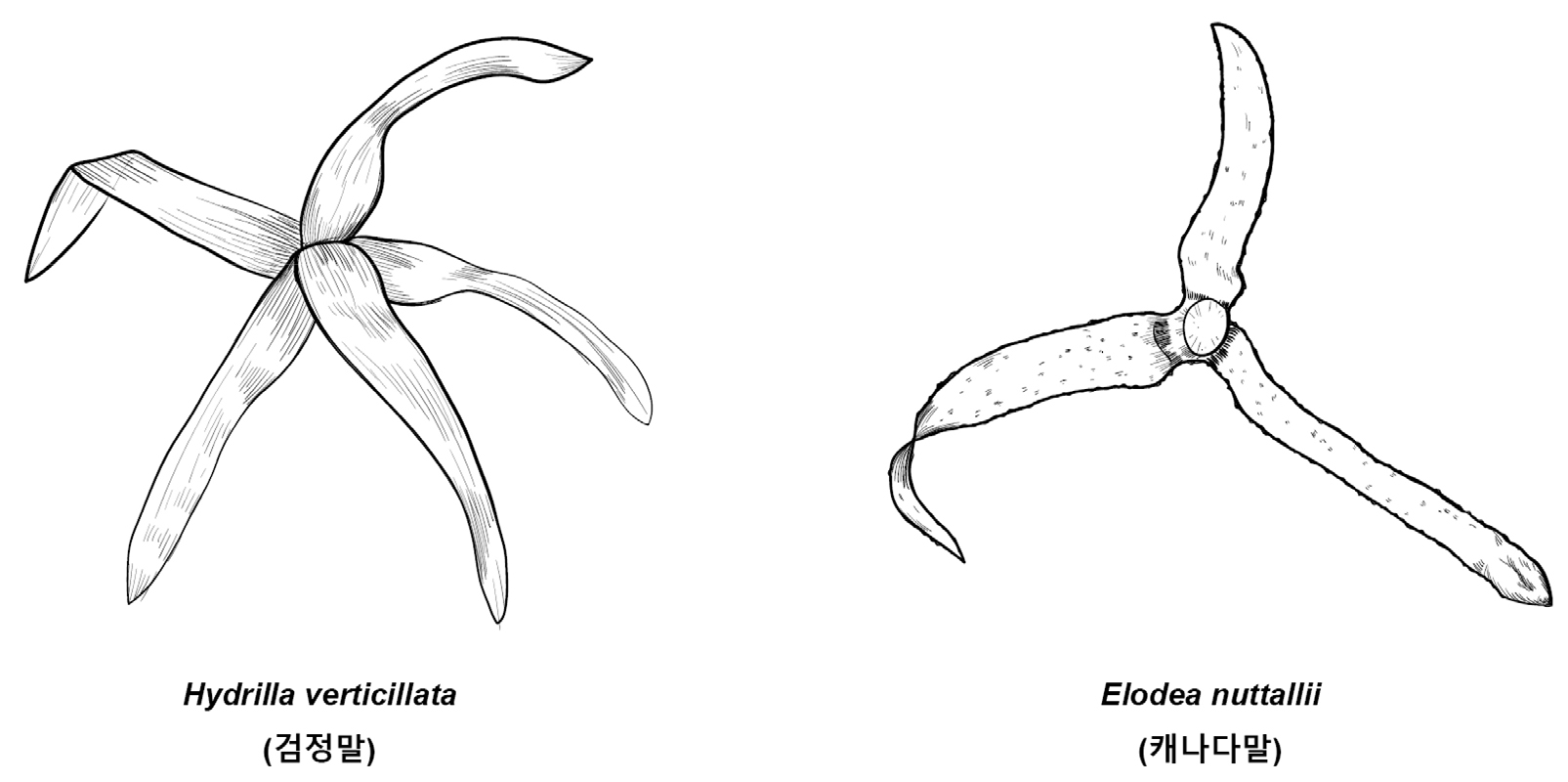
Key compared with morphologically similar species of related Korean genera (Korean name) (Fig. 7)
1. 3 to 8 whorled leaves on stem Hydrilla verticillata (검정말)
1. Mostly 3 whorled leaves on stem Elodea nuttallii (캐나다말)
Harmfulness and characteristics of weeds
E. nuttallii grows well in freshwaters and dries to death when out of the water. It grows very fast and reproduces vegetatively through fragments and is easily dispersed by water flow, which is the characteristic of the successful invader. For example, in Japan, all individuals that were discovered by 1984 have staminate flowers, so they have to spread rapidly through vegetative reproduction (Kunii, 1984). There are several adverse effects of the species: obstruct the movement of boats and fishing to a depth of 3.2 meters, spoil the beauty, have a bad influence on inhabitation of native plants and marine invertebrates because of eutrophication in a condition of its concentrated inhabitation. Its habitat is underwater such as the lake, reservoir, ponds, etc., thus, it is difficult to apply chemical and physical controls. This plant occurs in California between -5.1℃ to 9.7℃ in annual temperature, 6.4℃ to 23.5℃ in summer, the elevation of 150 m to 3,247 m and 332 ㎜ to 1,791 ㎜ in annual precipitation. If this species would be introduced in the Korean peninsula, it is highly possible to spread out all the area rapidly.
Weed risk assessment
By the result of weed risk assessment criteria (Animal and Plant Quarantine Agency, 2016), the degree of risk is 25% (Table 2). We suggest that this species could be treated as an ordinary weed.
Hydrocharis morsus-ranae L., Sp. Pl. 2: 1036 (1753)
Korean Name : Yu-reop-ja-ra-pul (유럽자라풀; Ministry of Environment, 2019).
Common Name : European frog-bit, Common frog-bit, Frog-bit, Water prog-bit, Water-poppy.
Taxonomic description and distribution characteristics
Taxonomic description: Perennial aquatic herbs, it can grow 0.1 to 1.3 m in diam. because of stolon. Each rosettes 1-30 ㎝ long. Unbranched roots with numerous root hairs, up to 50 ㎝ long, change color from green to white as they develop. No rhizomes, solid stolon has winter bud in autumn. Floating simple leaves, leaf blade circular or cordate, 1.2-6.0 ㎝ long and 1.3-6.3 ㎝ wide, entire, indented base, the under surface of a leaf is dark purple; underneath midribs with vivid aerenchyma, all veins arising from the base; slender petioles, 6-14 ㎝ long, with two free lateral stipules up to 2.5 ㎝ long at the petiole base. Monoecious. Staminate flowers with spathe composing 2 bracts; each bract 1-1.2 ㎝ long and 0.7-5.5 ㎝ wide; spathes with 1 to 5 staminate flowers, pedicel 4 ㎝ long; sepal greenish white; petal 9-19 ㎜ long, broad obovate to ovate, white; 4 whorled stamen 9 to 12, 2-3.5 ㎜ long, with the two outer whorls being fertile, and the inner whorls sterile or partly sterile. Pistillate flower peduncles up to 9 ㎝ long; sepal greenish white, 4-5 ㎜ long; petal 10-15 ㎜ long, obovate to ovate, white to pink, staminode bifurcate; stigmas 6, approximately 5 ㎜ long, divided to 1/4 – 2/3 of their length. Berry with 26 to 42(-about 70) seeds; seeds about 1-1.3 ㎜ long, transverse section oval, spiral pattern on the outer surface (Cook and Lüönd, 1982; Flora of North America Editorial Committee, 1982). Chromosome number 2n=28 (Catling et al., 2003).
Growing conditions: This species prefers low-flowing shallow water and occur in riverside, lakes, ponds, and open wetlands, as well as in waterways, marshes and irrigation channels (Fig. 8). It tolerates a wide range of climatic conditions. Organic matter is necessary for this species to grow. In other words, it is difficult to grow in an environment where inorganic substance is abundant like mud.
Origin: Europe, temporary areas of Asia and Africa. Some scholars limit the origin of this species to western and central Europe.
Invaded areas: This species is introduced in North America, especially, southeastern Canada and eastern USA (POWO, 2019).
Current state of designations: In USA, this species was designated as a toxic weed (USDA-NRCS, 2020) in some regions.
Specimens observed
[CM] 251973 (Ivan Nielsen, 1971. 8. 6. Denmark); 296010 (J.E. Charlebois, 1967. 7. 3. Canada) [CONN] 00067683 (Nicholas Tippery, 2004. 8. 15. United States); 00070303 (Leslie J. Mehrhoff, 2003. 8. 14. United States); 00097036 (Nicholas P. Tippery, 2009. 8. 17. United States) [LD] 1100673 (Åke Svensson, 2005. 7. 30. Sweden); 1121496 (Åke Svensson, 2002. 8. 8. Sweden); 1254784 (B. Nilsson & C. Wigermo, 2004. 8. 17. Sweden); 1926806 (Marie-Anne Larsson, 2005. 8. 14. Sweden) [TEX] A.A. Reznicek 12577, 2019. 9. 19. United States.
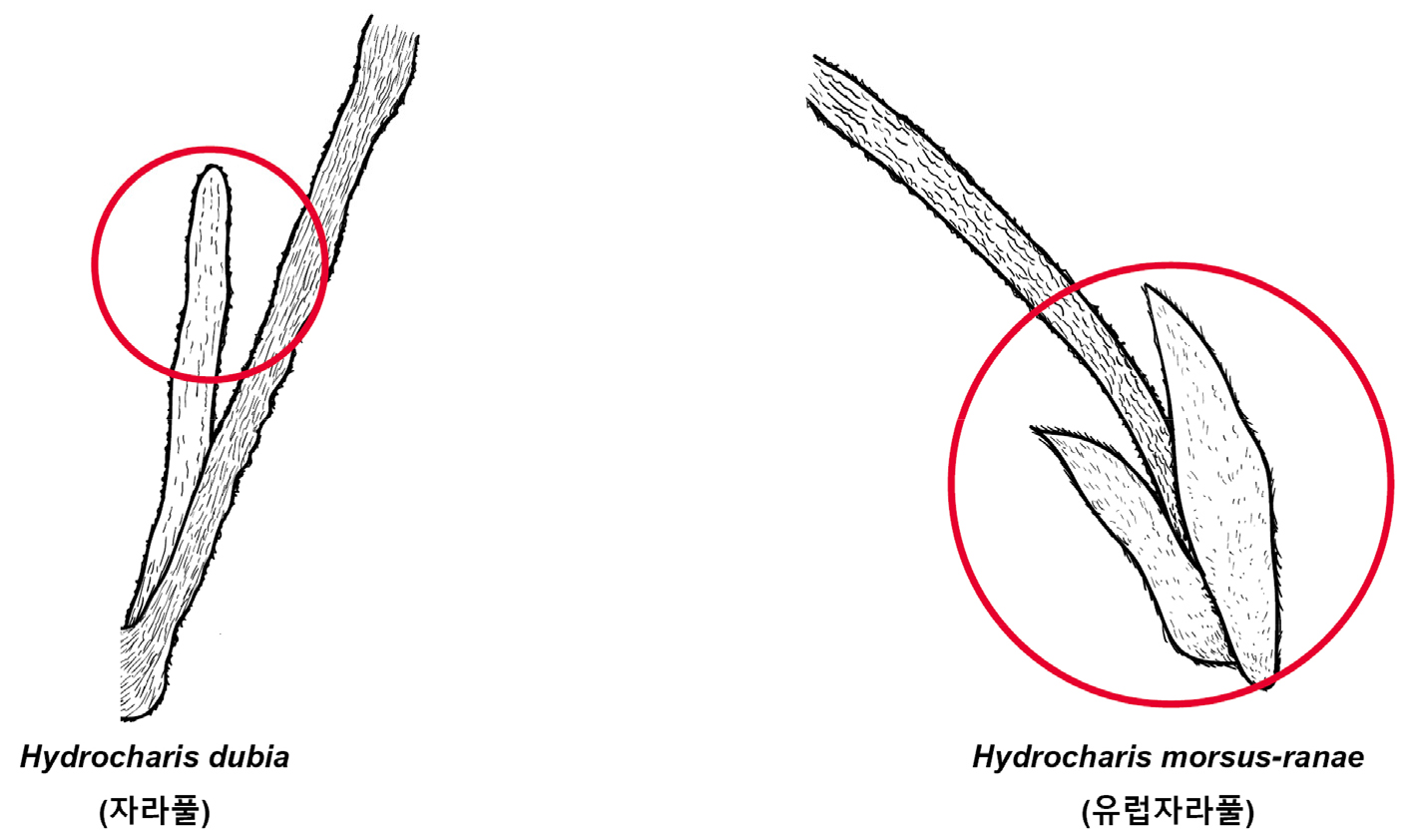
Key compared with morphologically similar species of related Korean genera (Korean name) (Fig. 9)
1. Plants above 20 ㎝ height, only one stipule dangles on the apex of petioles, leaf 2.8 to 8.0 ㎝ in diam. Hydrocharis dubia (자라풀)
1. Plants up to 20 ㎝ height, two stipule dangle on the base of petioles, leaf 1.2 to 4.4 ㎝ in diam. Hydrocharis morsus-ranae (유럽자라풀)
Harmfulness and characteristics of weeds
This species is a popular species as an aquarium plant. As a result, in many cases, it was intentionally spread out of its natural habitat. This plant has stolon as floating-leaved aquatic plants, which form a wide and dense mat, propagates vegetatively (rarely sexual reproduction) (Catling et al., 2003). H. morsus-ranae affects harmful influence environmentally and economically: native species disappear, reduce biodiversity, reduce water flow, block irrigation pumps, and disrupt leisure activities, thereby lowering aesthetic value. If this plant would be introduced in Korean ponds, reservoirs, and four major rivers (Hangang River, Nakdonggang River, Kumgang River, and Yeongsangang River), it is likely to reduce biodiversity. Since there is little control, it is a species that must be blocked beforehand.
Weed risk assessment
By the result of weed risk assessment criteria (Animal and Plant Quarantine Agency, 2016), the degree of risk is 43% (Table 2). We suggest that this species could be treated as an ordinary weed.
Stratiotes aloides L., Sp. Pl. 1: 535 (1753)
Korean Name : Chong-geom-ja-ra-pul (총검자라풀; Ministry of Environment, 2019).
Common Name : Water soldiers, Water pineapple, Saw tooth, Water aloe.
Taxonomic description and distribution characteristics
Taxonomic description Perennial, aquatic plant, 15 to 50 ㎝ height. Roots up to 180 ㎝, loose. Stems intensely short that it does not appear outward, the length of the reduced conical is 10-18 ㎜, producing leaves regularly, though complicated. Simple leaves with pointed apex, linear or lanceolate; submerged leaves 60 ㎝ long (rarely 110 ㎝), 1 ㎝ wide, thin and fragile, drooping obliquely, with relatively weak prickles at the edges; emerged leaves under 40 ㎝ long, 1-4 ㎝ wide, thick and sturdy, deep green, with clear prickle at the edges. Dioecious (some mentioned hermaphrodite flowers); many staminate flowers; pistillate flower only one, sepals 3, 3 petals tinged with white or pink, pistil fused 6 carpels, styles 6, dichotomous stigma, staminodes 15 to 30, peduncles are shorter and flat than leaves and have serrations on the edges. Berry with about 24 seeds (Charlton and Posluszny, 1999; Cook and Urmi-König, 1983). Chromosome number 2n=24, 40, 48 (Cook and Urmi-König, 1983; Negodi, 1929).
Growing conditions: This species is usually found in quiescent and shallow water. It prefers in eutrophic and mesotrophic lakes mainly filled with mud or organic substances (Strzałek and Koperski, 2009). It has often been observed growing in protected bay areas of large lakes, ponds, ditches and waterways.
Origin: Northwest Asia, East and North Europe.
Invaded areas: This species was naturalized in province of Ontario, Canada.
Current state of designations: Currently, various related organizations in Ontario are continuing to monitor and work to prevent the spread of this species. In USA, several states designate this species: ⅰ) in Alabama, as toxic weeds (Class C), ⅱ) in Florida, as prohibition aquatic plants (Class 1), and ⅲ) federal governments designate this species as a toxic weed list (USDA-NRCS, 2020). However, no particular danger has been reported at this time.
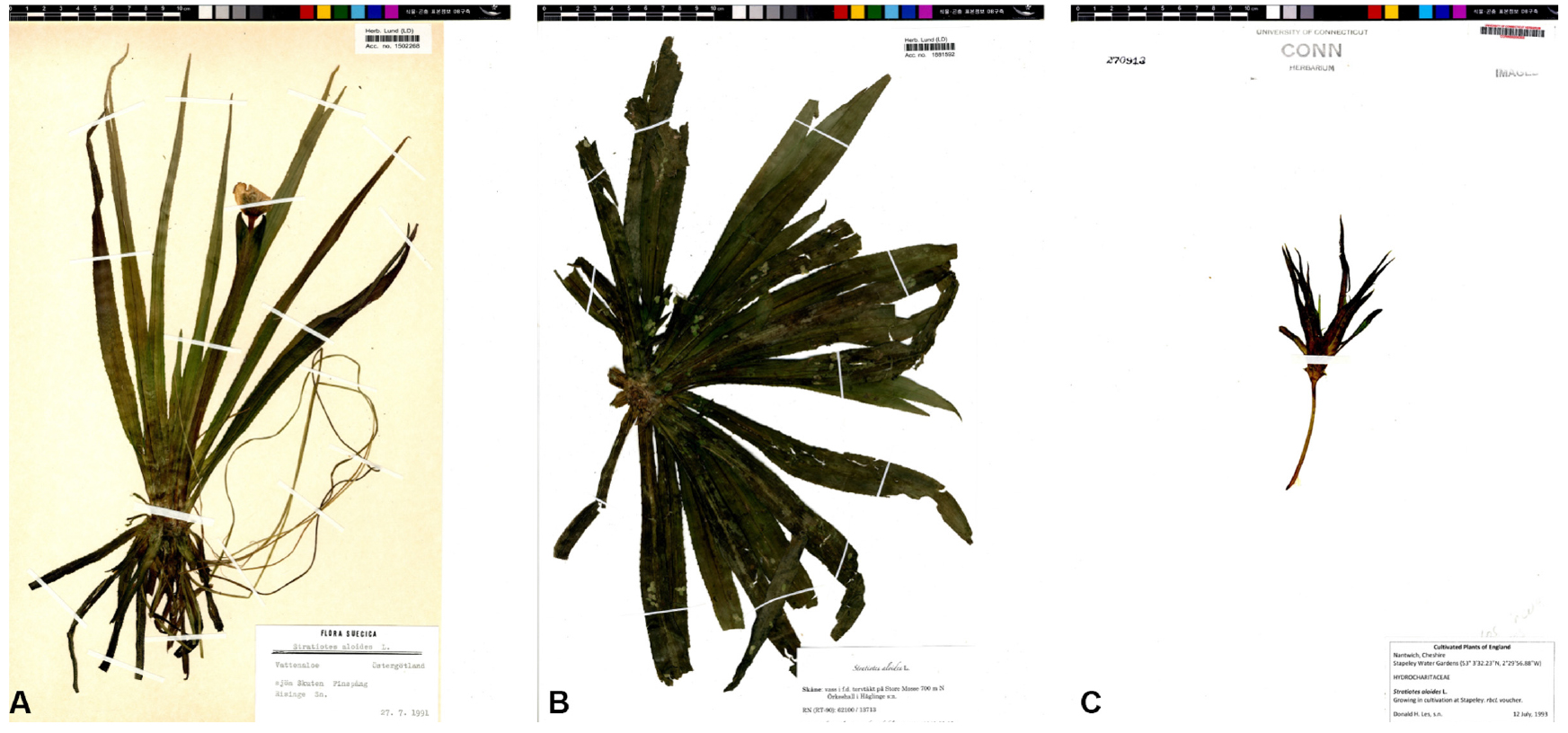
Specimens observed
[CONN] 00205303 (Donald H. Les, 1993. 7. 12. United Kingdom) [LD] 1502268 (Hans Zetterman, 1991. 7. 27. Sweden); 1881592 (?, 2013. 8. 18. Sweden) (Fig. 10).
Key compared with morphologically similar species of related Korean genera (Korean name) (Fig. 11)
1. Leaves rounded, oblanceolate, ovate or cordiform; petiolate 2
2. Stolon absent, leaves oblanceolate, cordiform Ottelia alismoides (물질경이)
2. Stolon present, leaves rounded, leaf bases cordiform Hydrocharis dubia (자라풀)
1. Leaves linear or lanceolate; sessile 3
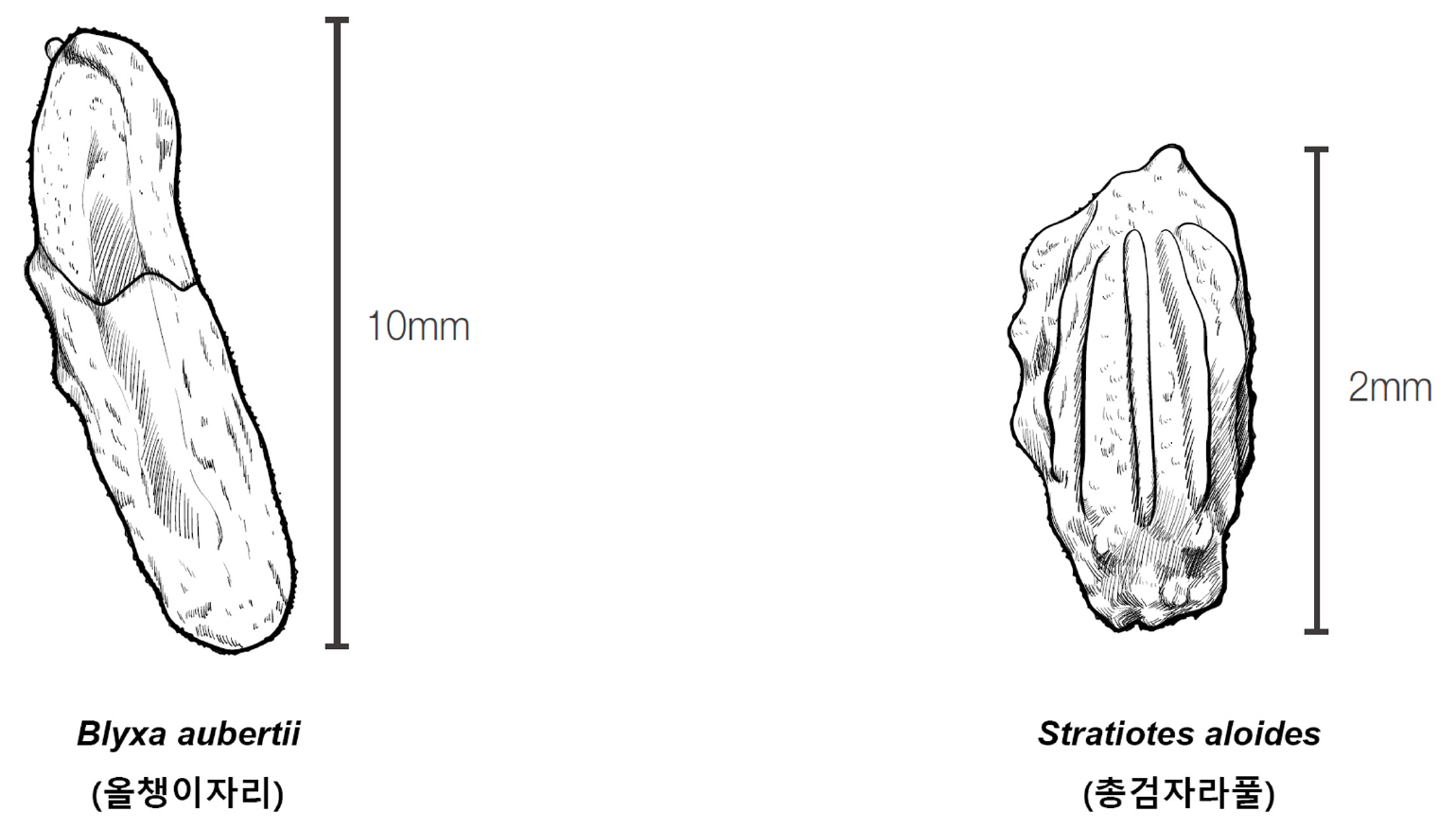
3. Leaves 7-30 ㎝ long, 3-7 ㎜ wide; fruits with 30 to 70 seeds; seeds within 2 ㎜ long Blyxa aubertii (올챙이자리)
3. Submerged leaves 60 ㎝ long, emergent leaves about 40 ㎝ long and 10-40 ㎜ wide; fruits with approximately 25 seeds; seeds about 10 ㎜ long Stratiotes aloides (총검자라풀)
Harmfulness and characteristics of weeds
This species covers the water surface and has serrated tooth-like structures on the leaves that interfere with leisure activities such as boating, fishing, swimming, and so on. It adversely affects tourist industry because it generates a rotting smell to attract pollinators during flowering. It blocks the flow of water and increases chemicals and precipitates, causing changes in the aquatic ecosystem (Mulderij et al., 2006). S. aloides propagates vegetatively and vegetative propagule appears an axil of leaves in the form of sprout. This sprout is separated, which can be floated in the water for more than 6 months and dispersed a long distance by water when bottom leaves of rosettes are decomposed (Sarneel, 2013). For this reason, this species can eliminate natural vegetation. Physical and chemical control is possible, but not effective. If this plant would be introduced in Korean ponds, reservoirs, and four major rivers, it is likely to reduce biodiversity.
Weed risk assessment
By the result of weed risk assessment criteria (Animal and Plant Quarantine Agency, 2016), the degree of risk is 52.5% (Table 2). We suggest that this species needs to be registered to control temporarily in law and to investigate the potential risk.
Eichhornia azurea (Sw.) Kunth, Enum. Pl. [Kunth] 4: 129 (1843)
Korean Name : Tacc-bu-re-ok-jam (닻부레옥잠; Ministry of Environment, 2019).
Common Name : Anchored water hyacinth, Rooted water hyacinth, Saw-petal water hyacinth.
Taxonomic description and distribution characteristics
Taxonomic description: Perennial floating aquatic plant, up to 100 ㎝ height. Rooted in mud, vary in length (5 ㎝ to 1 m). Vegetative stems raised at water surface, flowering stems with distal internode 2-10 ㎝, erect, 8-12 ㎝ long, glabrous. Leaves with petioles flat, stipule 7-13 ㎝ long, petiole 11-25 ㎝ long, leaf blade round, 7-16 ㎝ long and 2.3-16 ㎝ wide, on the surface; sessile leaves submerged, alternate in growing stem. Inflorescence spike with 7-50 flowers, each flower blossoms only 1 day; spathe obovate, 3-6 ㎝ long; peduncle with orange pubescents, 1.9-15 ㎝ long; perianth blue or white, corolla limb lobes obovate, 13-25 ㎜ long, margins rough, central lobe dark blue at base and yellow spot (Haynes, 1988); basal stamens 15-29 ㎜ long, outside stamens 6-20 ㎜ long; anthers 1.2-2.3 ㎜ long; style trifurcation. Fruits with 10 to 13 seeds. Seeds with ribs, 1.5-2.6 ㎜ long, 0.3-0.9 ㎜ wide (Flora of North America Editorial Committee, 1982) (Fig. 12).Chromosome number 2n=32 (Cook, 1998).
Growing conditions: This species commonly occurs in areas with water for a long time and up to alt. 1,000 m, but grows well in sludgy areas along rivers, lakes and wetlands, and prefers open areas with the slow flow of water.
Origin: The neotropical regions of South America.
Invaded areas: Bangladesh, Florida of USA (POWO, 2019).
Current state of designations: This species is designated as a federal noxious weed in the United States, and in many parts of the USA it is designated as an aquatic noxious weed by various criteria, including Class A, Prohibited, Quarantined and Prohibited aquatic plant Class 1 (USDA-NRCS, 2020). In Australia, it has been designated as a noxious weed on standard such as Noxious weed (Class 1) and Pest plant (Class 1), which is also applicable in Republic of South Africa. Shipping of this species estimated Regulated Weed Seed is also prohibited in New Zealand (Champion and Clayton, 2001).
Specimens observed
[CM] 335258 (Sue A. Thompson, 1988. 4. 10. Panama); 422849 (J. M. Duque Jaramillo, 1945. 12. 15. Colombia); 422850 (Herbert. H. Smith, ?, United States); 489712 (Hopkins, M.J.G., K.F. Rodrigues, E.S. Silva R. Pereira de Lima, J. Guedes de Oliveira & B. Lowy, 1986. 6. 19. Brazil).
Key compared with morphologically similar species of related Korean genera (Korean name) (Fig. 13)
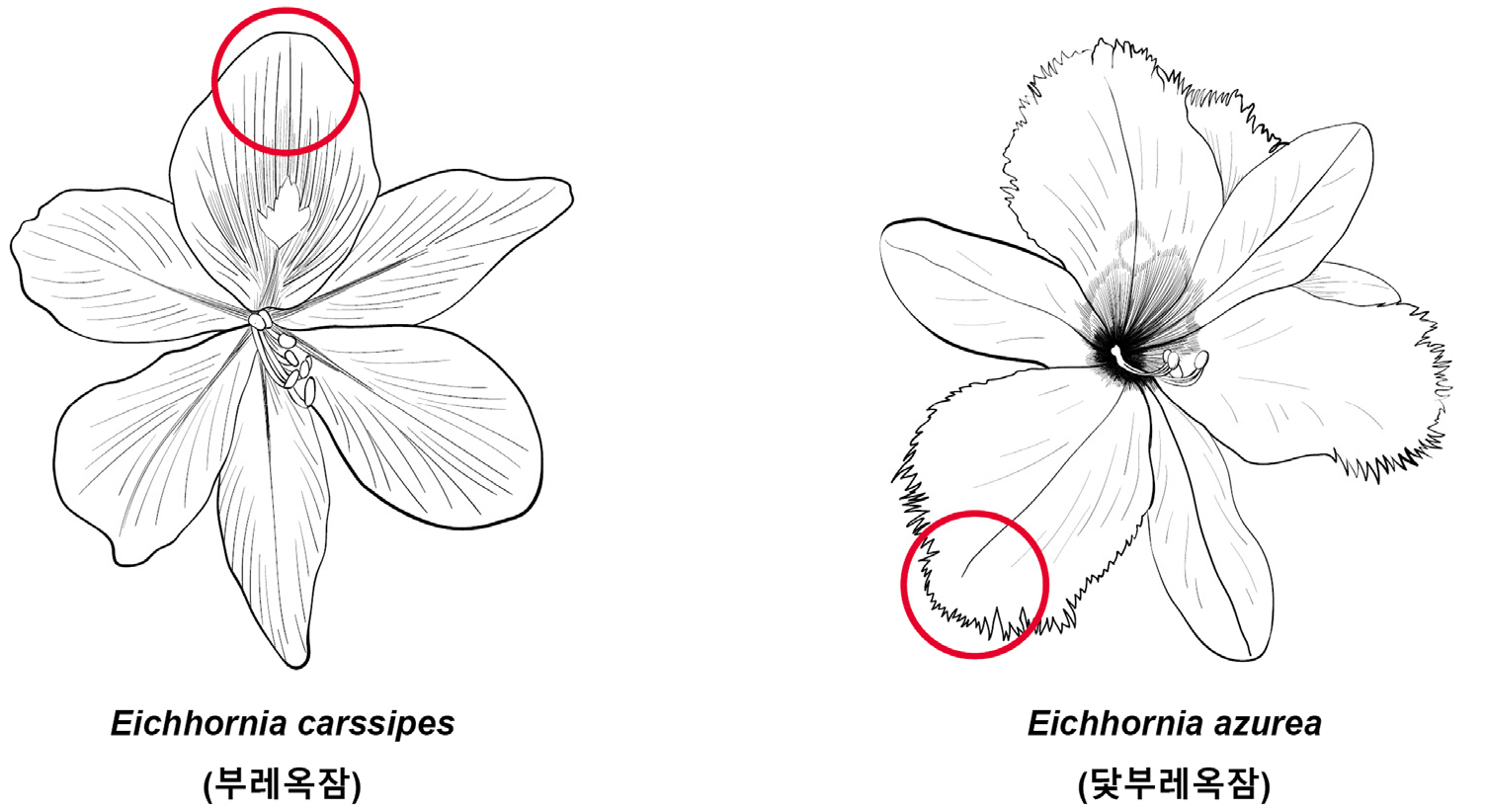
1. Floating aquatic plant, rosette of sessile, petioles of petiolate leaf inflate, edge of perianth is smooth Eichhornia carssipes (부레옥잠)
1. Rooted in mud, alternate sessile on elongate stem, petioles of petiolate leaf don’t inflate, edge of perianth is rough Eichhornia azurea (닻부레옥잠)
Harmfulness and characteristics of weeds
This species quickly forms an inflatable mat that completely covers a wide lake and floats on the water. It covers the surface of the water and drains the oxygen in the water, which greatly degrades the water quality of other aquatic habitats. It hinders tourism and causes economic losses such as fishermen and commercial transportation. This species was intentionally introduced because of its ornamental value, and in the USA, it is purchased for use in aquatic botanical gardens or aquariums, and advertised on commercial websites (Gopal, 1987; Kay and Hoyle, 2001). It is a plant that spreads through vegetative and seed propagation, and it grows large and well, and spreads in the mat over a wide area. It prefers tropical rain forests with over 60 ㎜ of precipitation per month and a tropical monsoon climate. The annual average temperature is 24-30℃, the minimum average temperature is 13-21℃, and the absolute minimum temperature is 0℃. There is no such region in Korea, but global warming can cause the invasion of parts of Jeju Island.
Weed risk assessment
By the result of weed risk assessment criteria (Animal and Plant Quarantine Agency, 2016), the degree of risk is 39% (Table 2). We suggest that this species could be treated as an ordinary weed.
Monochoria hastata (L.) Solms, Monogr. Phan. [A.DC. & C.DC.] 4: 523 (1883)
Korean Name : Hwa-sa-rip-mu-rok-jam (화살잎물옥잠; Ministry of Environment, 2019).
Common Name : Hastate-leaved pondweed, Arrowleaf false pickerelweed, Arrow-leaf monochoria.
Taxonomic description and distribution characteristics
Taxonomic description: Perennial aquatic plant. Rooted in mud. Vegetative stem often long and sturdy, flowering stem straight or slant, 50-90 ㎝ long. Radical leaves with sheath broaden base; petiole 30-90 ㎝; blade deltoid or triangular ovate, 5-15(-25) ㎝ long and 3-15 ㎝ wide, base sagittate or hastate, apex acute or acuminate. Inflorescences with 10 to 40 flowers umbel or short raceme, erect, remain after flowering; peduncle shorter than associated leaf petiole, pedicel 1-3 ㎝ long; perianth with greenish main vein, blue, red speckles, ovate, 1-1.6 ㎝ long; stamens 6, one of them is more than twice as long as the rest, elongated stamen: anthers 5.3-6.5 ㎜ long, shorted stamen: anthers 3-4 ㎜ long and filose filaments; apex with dense short hairs at apex. Capsule oblong, about 1 ㎝ long. Seeds with ca. 10 weak wings, oblong, brown, approximately 1 ㎜ long and 0.5 ㎜ wide (Flora of China Editorial Committee, 1994) (Fig. 14). Chromosome number n=17, 40, 42 (Eckenwalder and Barrett, 1986).
Growing conditions: As tropical aquatic plants, this species occurs in fresh water such as pools, rice fields and ditches within altitude of 700 m.
Origin: Oceania, Southeast Asia and south of China.
Invaded areas: Pakistan and Fiji (Mubarak et al., 2018; POWO, 2019).
Current state of designations: This species is a tropical aquatic plant of limited origin and is treated as a paddy weed for vegetative and seed propagation in Southeast Asia. External invasion of this species has been reported only in Singapore, but details are not known. Therefore, not only is there no known invasion of the natural vegetation of this species, but there is no information about its impact on biodiversity. Although it does not currently appear in the USA, the U.S. federal government designates it as a noxious weed in view of its potential to harm agriculture (USDA-NRCS, 2020). The northern region of Australia has been classified as Vulnerable due to concerns about invasion of this species and degeneration of aquatic environments.
Specimens observed
[CANB] 304023 (C.R. Dunlop & L. Craven, 1981. 4. 3. Australia); 723263 (S.W.L. Jacobs & K. Wilson, 1987. 6. 3. Australia) [LD] 1565542 (Håkan Wittzell, 2010. 2. 27. Cambodia).
Key compared with morphologically similar species of related Korean genera (Korean name) (Fig. 15)
1. Flowering stem usually 12-35 ㎝; bended inflorescence after flowering; leaves wide-lanceolate or triangular-ovate, 1.5-4 ㎝ wide Monochoria vaginalis (물달개비)
1. Flowering stem usually 30-200 ㎝; erected inflorescence after flowering; leaves wide ovate-cordiform, ensiform or triangular-ovate, 3-15 ㎝ wide 2
2. Leaf blade wide ovate-cordiform Monochoria korsakowi (물옥잠)
2. Leaf blade arrow-shaped or triangular-ovate Monochoria hastata (화살잎물옥잠)
Harmfulness and characteristics of weeds
This species is highly adaptable to freshwater environments in reservoirs where the flow rate is not high. It penetrates and diffuses over rice paddies, ponds, lakes, and rivers. It covers the surface of the water and blocks sunlight. Therefore, it drops the concentration of oxygen in the water and severely deteriorates the water quality. It disrupts water recreation such as boat movement and fishing, causing damage to fishing activities and tourism. Since M. hastata is difficult to control by physical and chemical methods, fundamental blocking is the best method at the import and distribution stages. Growth of this species occurs in: ⅰ) tropical rain forests or tropical monsoon climates, and ⅱ) annual temperature average is 25 to 35℃, the lowest temperature average is 16℃ (Useful Tropical Plants, 2019). In the Korea peninsula, there is no region with an average minimum temperature of over 16℃, but if global warming continues, there is a risk of penetration into some parts of Jeju Island.
Weed risk assessment
By the result of weed risk assessment criteria (Animal and Plant Quarantine Agency, 2016), the degree of risk is 43% (Table 2). We suggest that this species shall be treated as an ordinary weed.
Aegilops tauschii Coss., Notes Pl. Crit.: 69 (1849)
Korean Name : Ya-saeng-yeom-so-pul (야생염소풀; Ministry of Environment, 2019).
Common Name : Tausch’s goatgrass, Rough-spike hard grass.
Taxonomic description and distribution characteristics
Taxonomic description: Annual or biennial herb. Culms erect or curved, 20-40(-60) ㎝ high; usually close together. Leaf blades linear, 4-6(-17) ㎝ long, 2-6 ㎜ wide, rough, trichomes absent or rarely; sheaths glabrous, exterior sheaths pubescent; ligule 0.5-1 ㎜ long. Inflorescence cylindric spike, 5-10 ㎝ long (except awn), 5-13 fertile spikelet. Spikelet cylindric, ca. 9 ㎜ in diam., with 3-5 florets. Glume with 7-10 nerves 4-6㎜ long, apex flattened or dentated. Lemma lanceolate, with 5 veined; palea is equal in length to lemma. Awns of spikelet are shorter than spike. Caryopsis oblonged ovate, attached lemma and palea, 5-7 ㎜ long and width of 2 ㎜ or more, wrinkled, apex with light brown hairs (Fig. 16). Chromosome number 2n=14 (Flora of China Editorial Committee, 1994).
Growing conditions: This species occurs primarily everywhere. It is generally cold-resistant, as well as drought & salt resistant, and grows well in ochers, gravel paths, sandy areas, weed areas as well as semi-swamps.
Origin: Indian subcontinent, Caucasus, Ukraine (Eastern Europe).
Invaded areas: Europe (France, Germany, Greece, Italy, Nederland, etc.), North America.
Current state of designations: No records. This species is closely related to wheat, and a lot of wheat varieties have been produced in association with this plant. Therefore, this is an important plant from a molecular biological point of view.
Specimens observed
[CANB] 1007 (?, 1931. 4. 9. ?); 351578 (?, 1969. 2. 18. ?); 351590 (B.J. Lepschi, 1999. 8. 30. ?); 351591 (B.J. Lepschi, 1999. 8. 30. ?); 351592 (?, ?, ?).
Key compared with morphologically similar species of related Korean genera (Korean name) (Fig. 17)
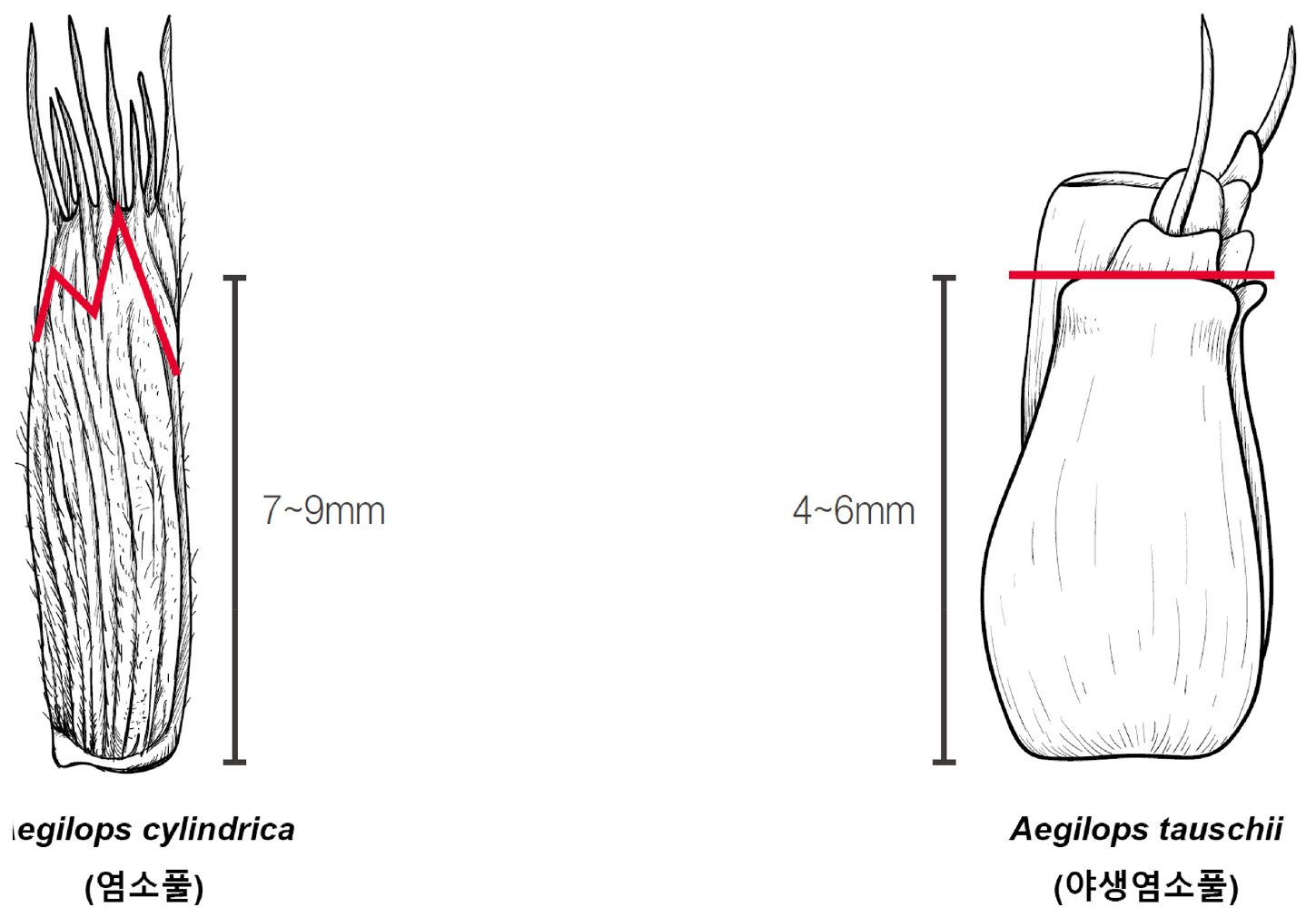
1. Glume 7-9 ㎜ long, not truncate, apex with 2 dentate; one is blunt, the other is short awn Aegilops cylindrica (염소풀)
1. Glume 4-6 ㎜ long, truncate, apex with short, blunt serrate Aegilops tauschii (야생염소풀)
Harmfulness and characteristics of weeds
This species is widely distributed in wheat tillage. The climate suitable for this species to grow is southeastern Europe and the Mediterranean region of northern Africa between 30° and 45° north latitude. This is a climate condition that spreads throughout the Korean peninsula. And this plant is resistant to dryness areas with an average annual rainfall of 150–350 ㎜. This species makes it easier to cross with cultivated wheat and reduces wheat productivity (Wang and Chen, 2019).
Weed risk assessment
By the result of weed risk assessment criteria (Animal and Plant Quarantine Agency, 2016), the degree of risk is 47.5% (Table 2). We suggest that this species could be treated as an ordinary weed.
Myriophyllum heterophyllum Michx., Fl. Bor.-Amer. (Michaux) 2: 191 (1803)
Korean Name : Mi-guk-mul-su-se-mi (미국물수세미; Ministry of Environment, 2019).
Common Name : Broadleaf watermilfoil, American water-milfoil, Variable watermilfoil, Two-leaf milfoil.
Taxonomic description and distribution characteristics
Taxonomic description: Perennial, aquatic herb, to 1 m. Hermaphroditic flowers; sometimes monoecious. Sturdy stem has crowded internode. Leaves are submerged or out of the water; submerged leaves are 4-5 whorled or scattered, dissected, oblong and 1.5-4 ㎝ long and 1-3 ㎝ wide; lobed in 5-12 pairs, filamentous, 0.5-1.5 ㎝ long (Fig. 18). Flowers are emergent; inflorescence spike of 4-whorled flowers. In monoecious, basal flowers are female but upper flowers are male. Recurved bracts are remained; lanceolate to oblong or obovate; 4-18 ㎜ long and 1-3 ㎜ wide. Stamens 4. Loments composed of 4 locules verge on globose, to 1.5 ㎜; apex beaked (Flora of China Editorial Committee, 1994).
Growing conditions: This species grows in lakes, ponds, rivers and swamps, and if it is isolated in places like mudflats, it is possible that it can live in semi-terrestrial form.
Origin: North America.
Invaded areas: Austria, Belgium, China Southeast, France, Germany, Great Britain, Netherlands and Spain (Cirujano et al., 1997; Dan et al., 2002; Hussner, 2012).
Current state of designations: This species was spread by vegetative propagation from cultivated areas. There is a record of the first influx from Germany in the 1960s in Europe (Stricker, 1962). In Spain, these plants became native plants (Cirujano et al., 1997), and were first identified in France in 2011 in a privately owned pond (Lebreton, 2013). In Asia, it is distributed in China (Dan et al., 2002), but the inflow route is uncertain. In Europe, it is listed on the EPPO list (EPPO, 2021). Also, this plant is listed as a noxious weed by the U.S. federal government or a state (USDA-NRCS, 2020).
Specimens observed
[TEX] George Yatskievych 18-112, 2018. 5. 6. United States.
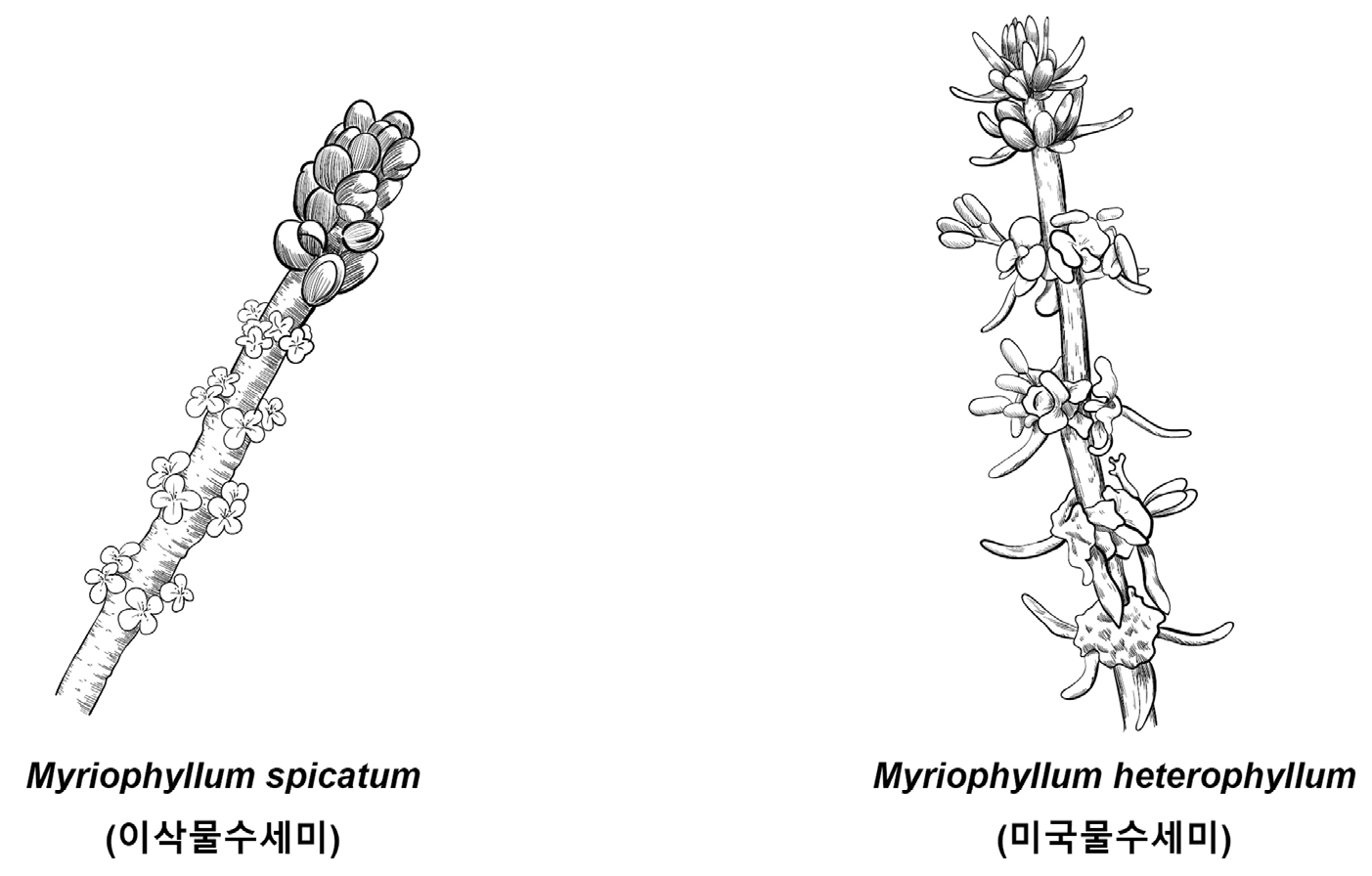
Key compared with morphologically similar species of related Korean genera (Korean name) (Fig. 19)
1. Emersed leaves pinnatedly divided Myriophyllum verticillatum (물수세미)
1. Emersed leaves entire 2
2. Leaves in emergent inflorescence absent Myriophyllum spicatum (이삭물수세미)
2. Leaves in emergent inflorescence present 3
3. Dioecious; staminate peduncle present Myriophyllum ussuriense (선물수세미)
3. Monoecious; staminate peduncle absent Myriophyllum heterophyllum (미국물수세미)
Harmfulness and characteristics of weeds
It is found in freshwater environments, and even small fragments of plants spread out rapidly. If this species invades ponds, reservoirs, and rivers in South Korea, the ecosystem will disturb and biodiversity will be highly decreased. Because of the dense growth on water surfaces of this plants, it can block the waterways which can obstruct recreational activities such as boating, fishing, and swimming. This species breeds fast and forms dense mats to reduce the growth of native aquatic plants. It also reduces water quality and prevents other aquatic animal’s inhabitation. It is mainly propagated vegetatively by humans, animals, and water flow. In addition, hybridization with related species is very frequent (Moody and Les, 2002), and there is a high possibility that new species with strong harmfulness will be generated. It is a popular aquatic plant in aquariums, so it is traded under a variety of common and scientific names. It is difficult to control chemical and physical methods and should be careful of the initial inflow.
Weed risk assessment
By the result of weed risk assessment criteria (Animal and Plant Quarantine Agency, 2016), the degree of risk is 49% (Table 2). We suggest that this species could be treated as an ordinary weed.
Bunias orientalis L., Sp. Pl. 2: 670 (1753)
Korean Name : Hok-gat (혹갓; Ministry of Environment, 2019).
Common Name : Turkish warty-cabbage, Warty-cabbage, Hill mustard, Turkish rocket.
Taxonomic description and distribution characteristics
Taxonomic description: Biennial or perennial herbs, semi-rosette hemicryptophyte. Tubular caudex. Stems 25-100 ㎝ tall, in some cases up to 1.5 m tall; apex ramified; multicellular glandular tubercles present except for the flowers. Simple leaves, bottom leaves have 2-10 ㎝ petioles; leaf blade up to 40 ㎝ long, ca. 14 ㎝ wide, lanceolate, rough dentate. Upper leaves no petioles, lanceolate or linear, dentate or entire. Inflorescence raceme. Fruiting pedicles 1-2 ㎝, straight, divaricate, slender. Sepals spreading, yellow, oblong, 2.5-3 ㎜ long and 1-1.5 ㎜ wide, glabrous. Petals yellow, obovate, 5-8 ㎜ long, 2-5 ㎜ wide; 6 stamens, tetradynamous, yellow filaments 1.5-3.5 ㎜ long, anther oblong, 0.8-1 ㎜ long. Fruit silicle, ovate or rarely oblong, tubular, 5-8 ㎜ long, 3-5 ㎜ in diameter, glabrous, lignification, pointed apex. Seeds with spiral wrinkles, stout round, 1-2 each fruit, 2-3.5 ㎜ in diam. (Flora of China Editorial Committee, 1994; Flora of North America Editorial Committee, 1982) (Fig. 20). Chromosome number 2n=14 (Flora of China Editorial Committee, 1994; Gupta, 2016).
Growing conditions: This species generally grows in the downtown area, farmland, roadside or meadow.
Origin: It is hard to define. The opinion about the origin of this species is various. According to experts, American highland or Caucasus, Southeastern Europe, West Siberia and Slovakia or Iran and Turkey (Jehlík and Slavík, 1968; Meusel et al., 1965).
Invaded areas: Currently, this species is widely distributed in temperate territory. It is naturalized in Europe except Mediterranean area and Iceland, West Asia, Russia, China and North America (Hultén and Fries, 1986).
Current state of designations: This plant is a naturalized species that has invaded several continents from its origin and is referred to in some areas as a noxious weed. In USA, it is recorded as L48(Ⅰ) and CAN(Ⅰ) (native status code) with simply exotic plant (USDA-NRCS, 2020). There are no contents about toxic weed.
Specimens observed
[LD] 1762626 (Bjørn Hjorth, 1977. ?. ?. ?); 1765250 (Yngve Larsson, 2003. 6. 4. Sweden); 2022253 (Marie-Anne och Yngve Larsson, 2008. 6. 4. Sweden) [TEX] Kelly Kearns s.n., 2019. 7. 17. United States.
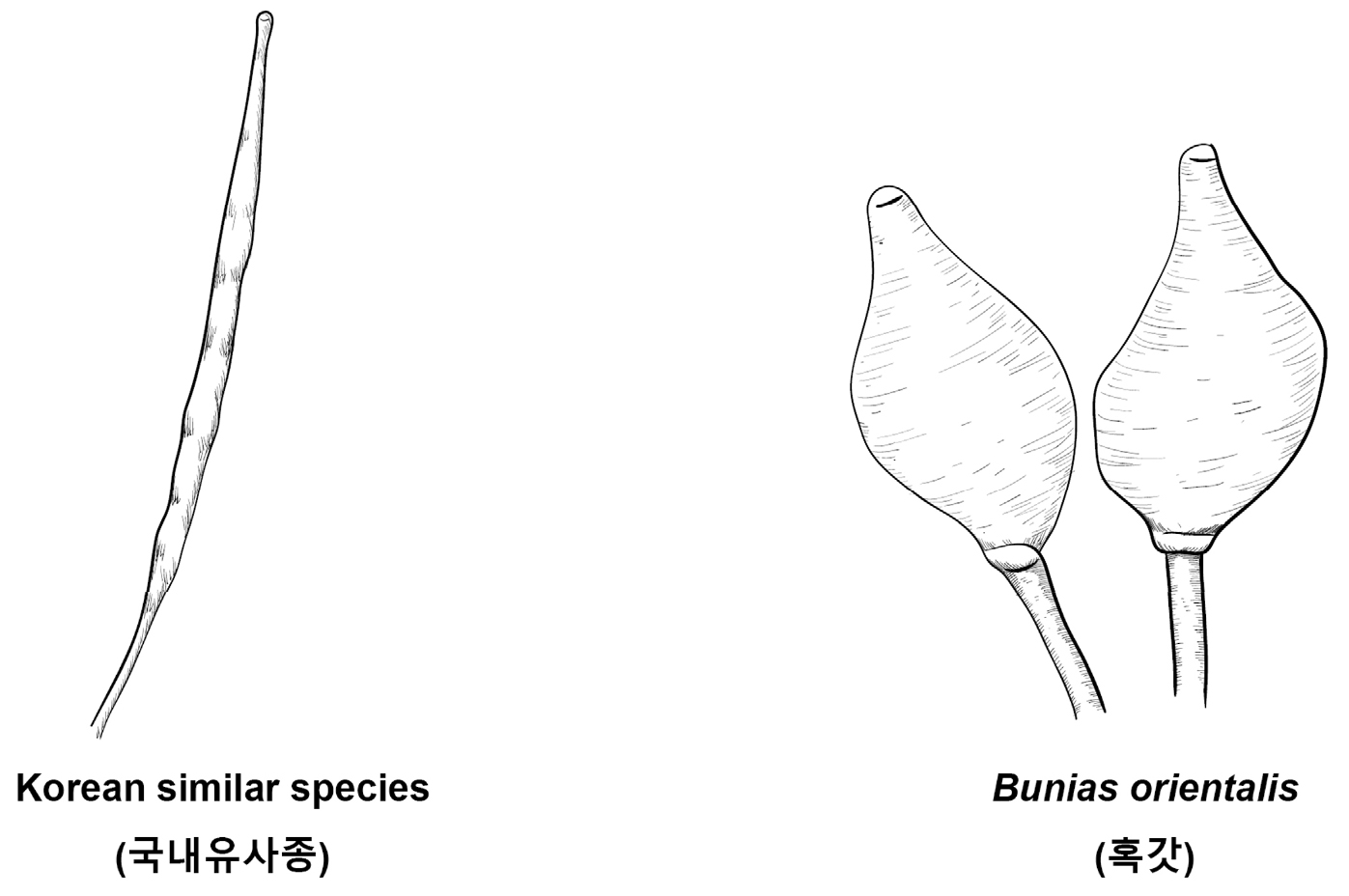
Key compared with morphologically similar species of related Korean genera (Korean name) (Fig. 21)
1. Silique, the length is longer than ten times the width 2
2. Stigma split a little or distinctly 3
3. Flowers with light red purple, stigma split a little, silique with 20-60 seeds Dontostemon dentatus (가는장대)
3. Flowers with yellow, stigma split into 2, silique with 90-130 seeds Sisymbrium luteum (노란장대)
2. Unbranched stigma 4
4. Cauline leaf base cover stems, fruits 5-11 ㎝ Brassica napus (유채)
4. Cauline leaf base not cover stems, fruits 3-5 ㎝ Brassica juncea (갓)
1. Silicle, the length is longer than twice the width Bunias orientalis (혹갓)
Harmfulness and characteristics of weeds
There are many introduction routes such as transportation with vehicles, cargo, and seeds of various crops, etc.; inflow of water and domestic animals, etc. (Jehlík and Slavík, 1968). This species propagates in a vegetative and generative means, so it is easily dispersed at tideland. Also, it has characteristics of simple seed fructification because of self-fertilization by bees or flies. B. orientalis has racemose inflorescence with many flowers in branched stems, therefore, it generates approximately 5,000 seeds per individuals and easily proliferate surrounding areas. As honey plants, this species attracts a large number of pollinators, which can cause difficulties in attracting pollinators to native plants growing around it. Growth of this species occurs in: ⅰ) regions with average temperature is -17 to 25℃, and ⅱ) temperate regions with annual precipitation of at least 500 to 3000 ㎜ (ISC, 2021). For these reasons, if introduced into the Korean peninsula, it is highly possible that this species may spread throughout all parts of Korea.
Weed risk assessment
By the result of weed risk assessment criteria (Animal and Plant Quarantine Agency, 2016), the degree of risk is 46% (Table 2). We suggest that this species could be treated as an ordinary weed.
Heracleum sosnowskyi Manden., Zametki Sist. Geogr. Rast. 12: 17 (1944)
Korean Name : Seo-yang-eo-su-ri (서양어수리; Ministry of Environment, 2019).
Common Name : Sosnowskyi’s hogweed, Cow parsley, Cow parsnip, Giant hogweed, Hogweed.
Taxonomic description and distribution characteristics
Taxonomic description: Biennial or perennial monocarpic plant. Hemicryptophyte with short rhizome. Height up to 3 m (usually 1-1.5 m); ridged stem with sparsely purple blotched trichomes. Trifoliolate leaves divided diversely, leaflets arranged in rows along the central petiole, apex glabrous, base with some hairs, margins with short rounded teeth. Inflorescence compound umbel with short hairy 30-75 rays, somewhat convex, 30 to 50 ㎝ in diam. Flower white or sometimes pinkish; outer petals radiate, 9-10 ㎜ long. Fruit schizocarp: 2 mericarps, ovate or elliptical, flattened, 9-16 ㎜ long and 5-9 ㎜ wide; unmatured mericarps with dense hairs; matured mericarps with wings, serrate along margin at apex, conspicuous vitta that do not reach the fruit base (until 3/4 of fruit) (Nielsen et al., 2005). Chromosome number 2n=22 (Bell and Constance, 1966).
Growing conditions: This species forms stand and occurs in pastures, mountain streams, flood plains of the lake. Also, it seems to prefer semi-natural habitat such as roadsides, disturbed areas, farmlands, parks and neglected orchards.
Origin: Eastern Caucasian ridge, south-western and eastern Transcaucasia (Georgia, Russia, Armenia, Azerbaijan and Turkey).
Invaded areas: Europe (Belarus, Hungary, Poland, Ukraine) and European parts of Russia (POWO, 2019).
Current state of designations: This species is registered as an invasive species in Europe (EPPO, DAISIE, NOBANIS) and is applied to several control programs to prevent further spread (USDA-NRCS, 2020). It produces 9,000 seeds per individual (Tkachenko, 1989) and can be easily sprayed by water over a long distance. On the other hand, it is recognized as a major toxic plant in Canada and placed on the Canadian weed seed list Class 2 (2016) (Justice Laws Website, 2021).
Specimens observed
[LD] 1954200 (Charlotte Wigermo, Åke Svensson, Bengt Nilsson, 2015. 8. 13. Denmark) (Fig. 22).
Key compared with morphologically similar species of related Korean genera (Korean name) (Fig. 23)
1. Height up to 1.5 m; mericarp 5-7 ㎜ long, oil ducts with slender and long claviform line reach 1/2 of mericarp Heracleum moellendorffii (어수리)
1. Height up to 3 m; mericarp 9-16 ㎜ long, oil ducts with bold and short claviform line reach 3/4 of mericarp Heracleum sosnowskyi (서양어수리)
Harmfulness and characteristics of weeds
H. sosnowskyi has a strong growth capacity and can occupy a large area at high density, creating competition with native species. In addition, species diversity decreases in the areas dominated by this species. If the skin is exposed to sunlight for more than 15 minutes after exposure to the sap emitted from plants, photosensitizing furanocoumarins contained the whole plant can burn and/or blister human skin (Jakubowicz et al., 2012). It affects the smell of meat and milk of animals that eat the plant body of this species as feed and may negatively affect the health of human beings who ingest it. Nevertheless, it is economically valuable (animal fodder or honey production) and easily propagated in a deliberate manner. In addition, it produces many seeds and propagates easily, so special care is needed. It is native to the Caucasus and Turkey (POWO, 2019), and is highly adaptable to low temperatures, so if it flows into Korea, it can spread all over the country.
Weed risk assessment
By the result of weed risk assessment criteria (Animal and Plant Quarantine Agency, 2016), the degree of risk is 69% (Table 2). We suggest that this species should to be registered to control in law.
Table 2.
Result of weed risk assessment for 11 species (Notice 2016-68, Animal and Plant Quarantine Agency)
zsp.1 Spirodela punctata (G.Mey.) C.H.Thomps., ysp. 2 Sagittaria graminea Michx., xsp. 3 Elodea nuttallii (Planch.) H.St. John, wsp. 4 Hydrocharis morsus-ranae L., vsp. 5 Stratiotes aloides L., usp. 6 Eichhornia azurea (Swartz) Kunth, tsp. 7 Monochoria hastata (L.) Solms, ssp. 8 Aegilops tauschii Coss., rsp. 9 Myriophyllum heterophyllum Michx., qsp. 10 Bunias orientalis L., psp. 11 Heracleum sosnowskyi Manden.