Introduction
Materials and Methods
Plant material and in vitro proliferation
Determination of suitable tissue for cryopreservation
Cold hardening and preculture procedure for cryopreservation
Loading and dehydration procedure for cryopreservation
Unloading procedure and plant regrowth
Data collection and statistical analysis
Results and Discussion
Comparison of the apical shoot tips and axillary buds for cryopreservation
Optimal conditions of plant material for cryopreservation by droplet-vitrification
Cryopreservation by droplet-vitrification
Introduction
Pear (Pyrus spp.) belongs to the genus Pyrus (P.), in the family Rosaceae (Potter et al., 2007). It is one of the most economically important fruit crops grown in the temperate regions (Li et al., 2022; Silva et al., 2014). There are at least 22 known Pyrus species. About 5,000 different pear accessions have been recognized and maintained throughout the world (Hong et al., 2021; Li et al., 2022). According to their original distribution, Pyrus is categorized into two major groups, European and Asian pears. Among Pyrus species, P. communis is predominantly cultivated in Western countries, whereas Asian pears are mostly represented by P. pyrifolia, P. bretschneideri, and P. ussuriensis that are distributed and grown in East Asian countries including Korea, China, and Japan (Bell, 1991; Hong et al., 2021; Li et al., 2022). Although there are diverse pear varieties, a relatively small number of them are extensively distributed and cultivated on a large scale (Quinet and Wesel, 2019). In recent decades, plant genetic diversity has been greatly reduced due to crop uniformity and genetic erosion resulting from various factors, including pathogens and pests as well as environmental changes. As a result, less profitable genetic resources are abandoned (Roque-Borda et al., 2021).
The conservation of pear germplasm, which is primarily propagated vegetatively, predominantly relies on field preservation (Yi et al., 2017). However, field conservation has risk of loss caused by disease, particularly the spread of fire blight pathogen among pear trees and/or unpredictable climate conditions. Also, in vitro conservation methods are susceptible to contamination and the occurrence of somaclonal variation (Chen et al., 2011; Towill, 1988), although they offer alternatives. Therefore, an efficient conservation system is needed to increase their utilization in the future and safely manage and preserve them without loss.
Cryopreservation has been studied as a method for long-term preservation of biological materials. In the case of pyrus species, P. cordata and Pyrus spp. by two step-freezing (Chang and Reed, 2000; 2001), Pyrus spp. by vitrification (Tahtamouni and Shibli, 1999), Pyrus spp. by encapsulation-dehydration (Yi et al., 2017; Zamecnik et al., 2007), P. pyraster by encapsulation-dehydration (Condello et al., 2009), and P. pyrifolia by encapsulation-dehydration (Hao et al., 2005) have been reported. Among several cryopreservation techniques, droplet-vitrification (Kim et al., 2007; Leunufna and Keller, 2003) is one of the recent techniques continuously adopted in clonally propagate plants. Droplet-vitrification has been applied to woody fruit species, including apple (Condello et al., 2011; Li et al., 2015), Prunus spp. (Ruzic et al., 2013; Vujovic et al., 2015), Citrus spp. (Volk et al., 2016), and Vitis spp. (Bi et al., 2018; Pathirana et al., 2016). However, pear cryopreservation has not been widely studied by droplet-vitrification (Guyader et al., 2019).
Optimizing cryopreservation protocols is essential for ensuring the preservation of genetic resources for pear accessions. The main purpose in the present study was to establish a successful cryopreservation method by droplet-vitrification using in vitro shoot tips in two pear cultivars, ‘Bartlett’ (P. communis) and ‘BaeYun No.3’ (P. pyrifolia).
Materials and Methods
Plant material and in vitro proliferation
One accession each of two Pyrus taxa, ‘Bartlett’ (P. communis L.) and ‘BaeYun No.3’ (P. pyrifolia Nakai), were used in this study for improvement of cryopreservation by droplet-vitrification method in Pyrus species. These pear cultivars were obtained from National Institute of Horticultural and Herbal Science (NIHHS) of the Rural Development Administration (RDA). In vitro propagated cultures of two Pyrus species were cultured on Murashige and Skoog (MS) medium (Murashige and Skoog, 1962) with 2.0 ㎎/L N6-benzyladenine (BA) and 0.2 ㎎/L indole-3-butyric acid (IBA) containing 30 g/L sucrose according to the method previously described by Yi et al. (2015) and Song et al. (2022). All cultures were maintained in a culture room at 24 ± 1℃ under a 16 h of photoperiod.
Determination of suitable tissue for cryopreservation
To determine suitable tissue parts for cryopreservation, we conducted a series of experiments to evaluate preculture (PC) and loading solution (LS) treatments using the apical shoot tip and axillary bud collections. Each of collected explants was precultured in a liquid MS medium with sequential sucrose concentrations of 0.3 M for 31 hours, and 0.7 M for 17 hours in a dark room at 25℃. Following preculture steps, collections were treated in a loading solution containing 35% of PVS3 (LS, C4) (Kim et al., 2009) for 40 min. After PC and LS treatments, we surveyed regrowth rates of shoot tips and axillary buds after culture on a hormone-free MS medium for six weeks. Based on results, we examined for development of pear cryopreservation technique using apical shoot tips only as shown in Fig. 2. Shoot tips under 2 ㎜ in length were used for the following experiments (Fig. 1D).

Fig. 1.
Optimal conditions of plant material for cryopreservation of pear (Pyrus spp.) by droplet-vitrification. (A), pear plants cultivated on propagation medium after six weeks of culture. (B), shoots on basal MS medium for cold treatment for 7 days at 4℃ after cutting from seedlings. (C), shoots ready for shoot tip excision after cold-hardening. (D), excised shoot tips, just under 2 ㎜ for cryopreservation.
Cold hardening and preculture procedure for cryopreservation
Shoots of approximately 1-2 ㎝ in length collected from propagated seedlings were cold hardened on a solid MS medium at 4℃ for 7 days in a dark room (Fig. 1B). Apical shoot tips collected from cold-hardening shoots were precultured in liquid MS medium containing 0.3 M sucrose for 31 hours and then treated with a liquid MS medium containing 0.7 M sucrose for 17 hours in a dark room at 25℃.
Loading and dehydration procedure for cryopreservation
Precultured shoot tips (MS + 0.3 M sucrose for 31 h and MS + 0.7 M sucrose for 17 h) were treated in a loading solution containing 35% of PVS3 (LS, C4) for 30, 40, or 50 min (Table 1 and Fig. 2B) and exposed to a dehydration solution (B1) containing plant vitrification solution (PVS3, 50% glycerol and 50% sucrose) (Kim et al., 2009) for 60, 90, or 120 min at 25℃ (Table 1 and Fig. 2C). Explants were transferred onto droplets consisting of 3 µL PVS3 on sterilized aluminum foils (4.0 × 0.5 ㎝) (Fig. 2D). They were then carefully immersed in liquid nitrogen (LN, -196℃) and kept for at least 60 min (Fig. 2E).
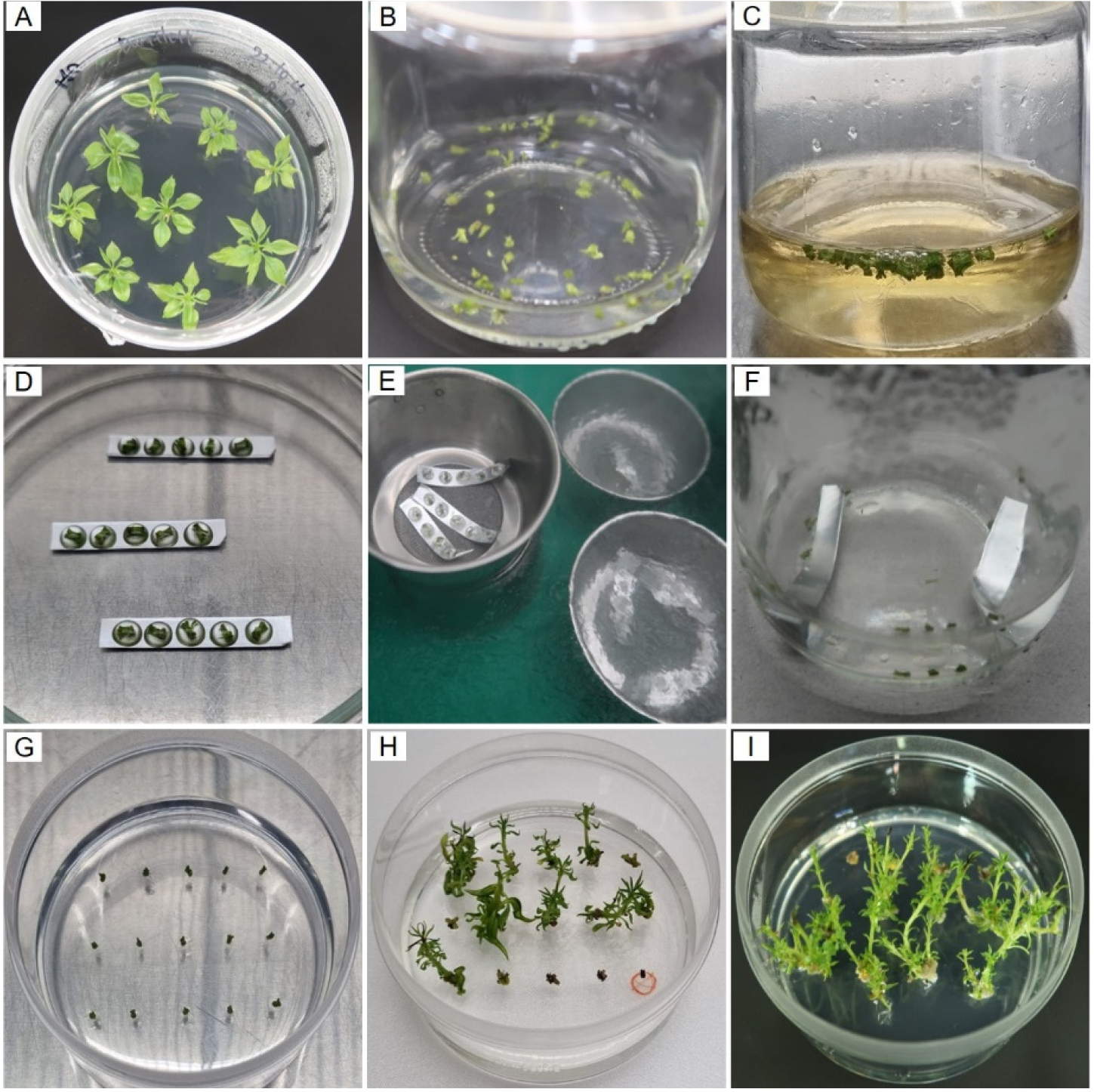
Fig. 2.
Experimental procedure of cryopreservation using shoot tips of pear (Pyrus spp.) by droplet-vitrification. (A), plantlets on basal MS medium for cold-hardening. (B), shoot tip collections from the plantlets are treated with loading solution (LS, C4) for 40 min at 25℃. (C), dehydrated with PVS3 solution (B1) for 90 min. (D), shoot tips in droplets of PVS3 solution on sterilized aluminum foil strips. (E), immersion of explants on the foil into liquid nitrogen (LN) for 1 h. (F), cryopreserved shoot tips on foil strips in pre-heated solutions (40℃), MS+0.8M sucrose, for 40 min for thawing. (G), unloaded shoot tips on recovery culture medium. (H) and (I), ‘Bartlett’ and ‘BaeYun No.3’ cultivars, plant regeneration from cryopreserved shoot tips on recovery culture medium for 8 weeks after cryopreservation.
Table 1.
Experimental procedures used for cryopreservation of Pyrus species and regrowth comparison of two pear cultivars cryopreserved (+LN) according to time exposed to LS and PVS3 by droplet-vitrificationz
|
Cryopreservation method | Preculture |
Osmoprotection (Loading solution, LS) |
Cryoprotection (PVS3, B1) |
Sample status | Cultivars | |
| Timing of application (min) | Bartlett | BaeYun No. 3 | ||||
| Regrowth rate (%) | ||||||
| #1 |
0.3M sucrose (31 h) + 0.7M sucrose (17 h) | 30 | 60 | Freshy | 93.3 ± 5.4 | 100 ± 0 |
| -LNx | 91.7 ± 3.9 | 100 ± 0 | ||||
| +LNw | 77.1 ± 3.9 abc | 33.3 ± 6.3 cd | ||||
| #2 | 90 | Fresh | 100 ± 0 | 100 ± 0 | ||
| -LN | 89.7 ± 2.1 | 97.2 ± 2.3 | ||||
| +LN | 75.6 ± 9.6 bc | 62.2 ± 4.8 ab | ||||
| #3 | 120 | Fresh | 100 ± 0 | 100 ± 0 | ||
| -LN | 88.9 ± 4.5 | 94.9 ± 2.1 | ||||
| +LN | 73.3 ± 5.4 c | 48.9 ± 7.9 abc | ||||
| #4 | 40 | 60 | Fresh | 93.3 ± 5.4 | 100 ± 0 | |
| -LN | 97.4 ± 2.1 | 84.8 ± 6.4 | ||||
| +LN | 93.3 ± 0.0 ab | 35.6 ± 4.8 bcd | ||||
| #5 | 90 | Fresh | 100 ± 0 | 100 ± 0 | ||
| -LN | 90.3 ± 5.1 | 97.2 ± 2.3 | ||||
| +LN | 95.6 ± 1.8 a | 68.9 ± 7.9 a | ||||
| #6 | 120 | Fresh | 100 ± 0 | 100 ± 0 | ||
| -LN | 91.7 ± 3.9 | 79.9 ± 2.4 | ||||
| +LN | 86.7 ± 3.1 abc | 40.0 ± 6.3 bcd | ||||
| #7 | 50 | 60 | Fresh | 100 ± 0 | 100 ± 0 | |
| -LN | 100 ± 0 | 92.3 ± 3.6 | ||||
| +LN | 95.4 ± 1.9 a | 35.6 ± 7.9 bcd | ||||
| #8 | 90 | Fresh | 100 ± 0 | 93.3 ± 5.4 | ||
| -LN | 91.8 ± 3.6 | 91.9 ± 3.9 | ||||
| +LN | 82.2 ± 3.6 abc | 45.6 ± 8.1 abc | ||||
| #9 | 120 | Fresh | 100 ± 0 | 100 ± 0 | ||
| -LN | 92.3 ± 6.3 | 84.0 ± 4.1 | ||||
| +LN | 77.8 ± 4.8 abc | 18.4 ± 4.1 d | ||||
zShoots excised from in vitro seedlings were cold hardened at 4°C for 7 days in a dark room. Apical shoot tips (2.0 ㎜ in length) were separated from the shoots prior to preculture. Precultured explants (MS + 0.3 M sucrose for 31 h and MS + 0.7 M sucrose for 17 h) were treated with a loading solution containing 35% of PVS3 (LS, C4) for 30, 40 or 50 min and exposed to dehydration solution (B1) containing PVS3 (50% glycerol + 50% sucrose) for 60, 90 or 120 min prior to direct immersion in liquid nitrogen (LN) for 60 min. Cryopreserved (+LN) explants were thawed in liquid MS medium with 0.8 M sucrose (40℃) for 30 s and transferred into liquid MS medium containing 0.8 M sucrose (RT). Thawed and non-cryopreserved (-LN) explants were cultured for regrowth in MS medium containing 3% sucrose, 1.0 ㎎/L BA, 0.1 ㎎/L GA3 (regrowth medium) for 8 weeks. Mean data with standard errors are shown. Means followed by the same letter are not significantly different (p > 0.05) according to Duncan’s multiple range test.
Unloading procedure and plant regrowth
Explants on foil strips in LN were rapidly soaked and thawed in 20 mL pre-heated unloading solution (40℃) consisting of liquid MS with 0.8 M sucrose (Fig. 2F). Half volume of the unloading solution was then immediately removed after 30 sec followed by adding 10 mL of another unloading solution (25℃) at room temperature (RT) for 15 min. These explants were transferred to 10 mL of fresh unloading solution (RT) for 25 min. All the explants were placed on MS medium without plant growth regulators (PGRs) as a control and on regrowth medium consisting of MS medium including 3% sucrose, 1.0 ㎎/L BA, and 0.1 ㎎/L gibberellin (GA3) for recovery after thawing treatments (Fig. 2G). Explants were then sub-cultured every two weeks.
Data collection and statistical analysis
Explants showed green shoot tips after 2 weeks of culture in the regrowth medium. Regrowth rate was then evaluated 8 weeks after transferring explant onto the regrowth medium by counting the number of shooting plants showing growth and developed leaves. Data were analyzed using the least significant difference (ANOVA) at p < 0.05 and Duncan’s multiple range test with SAS 7.1 software (SAS Institute Inc., Cary, NC, USA). Results are presented as mean percentages.
Results and Discussion
The aim of this study was to optimize cryopreservation protocols by droplet-vitrification method for Pyrus germplasm to ensure preservation of genetic diversity. Apical shoot tips of the two cultivars, ‘Bartlett’ and ‘BaeYun No.3’, were used to define how the duration of LS, PVS treatments and the type of regrowth medium affected regrowth of explants after cryopreservation.
Comparison of the apical shoot tips and axillary buds for cryopreservation
Various types of tissues such as cell suspensions, pollen, embryos, seeds, shoot tips and dormant buds can be used to cryopreservation. In the cryopreservation of vegetatively propagated species, the most generally utilized tissues are shoot tips and axillary buds of in vitro grown plantlets (Lambardi and De Carlo, 2003).
To determine the optimal tissue to be used for cryopreservation of Pyrus species, two tissue parts, apical shoot tips and axillary buds, were used and then applied under precultue (PC) and loading (LS) treatments. Effects of PC and LS treatments on the regrowth rates are shown in Fig. 3. When explants were not immersed in LN, shoot tips and axillary buds did not show reduced regrowth rate in Fresh (explants without treatment) or PC treatments for the two cultivars. However, after LS treatments in both cultivars, regrowth rates of axillary buds were significantly decreased: 64.4% for ‘Bartlett’ (Fig. 3A) and 56.7% for ‘BaeYun No.3’ (Fig. 3B). In the case of shoot tips, there was no difference in regrowth rate of shoot tips between Fresh and LS treatments (100% for ‘Bartlett’ and 93.3% for ‘BaeYun No.3’). In this experiment, the method of using apical shoot tips showed better regrowth rate results than using axillary buds (Fig. 3).

Fig. 3.
Effect of preculture and LS treatments on regrowth of shoot tips and axillary buds derived in vitro grown shoots from both cultivars, ‘Bartlett’ (A) and ‘BaeYun No.3’ (B). Error bars indicate SE. Bars marked with the same letter are not significantly (p > 0.05) different according to Duncan’s multiple range test.
Optimal conditions of plant material for cryopreservation by droplet-vitrification
There are various key factors to promote regeneration from cryopreserved explants, including growth stage, size, health of the donor material with good vigor (Hofer and Flachowsky, 2023), and cold hardening of plants in aspects of plant physiology conditions. In addition, Reed (1990) has reported that the age of the in vitro grown seedlings can considerably affects the regrowth rate from cryopreserved shoot tips. Optimal shoot tip size of vigorous seedling might be contributed to tolerance of dehydration and freezing and high regeneration rate following cryopreservation. Cold acclimation is very effective for improving the recovery rate of shoot tips in cryopreserved Pyrus, especially in temperate plants (Reed, 1998; Tahtamouni and Shibli, 1999). We used the following plant conditions prior to cryopreservation: 1) well-developed seedling of 5 or 6-week-old cultures from about 1.5 ㎝ shoots on the propagation medium (Fig. 1A); 2) cold-hardening of shoots of about 1-2 ㎝ in length excised from propagated seedlings (Fig. 1B); 3) the optimal size (2 ㎜) of apical shoot tips (Fig. 1D) obtained from the cold-hardening shoots (Fig. 1C). Previous researchers have reported that various sizes of shoot tips with a minimum of 0.8 ㎜ to a maximum 3 ㎜ in pear species (Chang and Reed, 2000; Hofer and Flachowsky, 2023; Niino et al., 1992; Scottez et al., 1992). Considering these results, apical shoot tips of vigorous plants with cold hardening were used for the following experiments, such as preculture, loading solution, and dehydration (Fig. 2).
Cryopreservation by droplet-vitrification
Determination of suitable and stable loading solution (LS, C4) and dehydration solution (PVS3, B1) is important for increasing efficiency of regrowth after treatment of LN by alleviate osmotic stress and toxicity (Benson et al., 1996; Rall and Fahy, 1985; Yamamoto et al., 2012). For this reason, the time of exposure to LS and PVS3 must be varied for experiments. To determine the optimal application times for LS and PVS3 treatments, precultured shoot tips were osmoprotected by LS and cryoprotected by PVS3 with nine cryopreservation methods (#1 to #9) for the two pear cultivars. Results are shown in Table 1. There was no negative impact or toxic effect of PC, LS and PVS3 treatments, 88.9%±4.5 to 100% for ‘Bartlett’ and 79.9%±2.4 to 100% for ‘BaeYun No. 3’. Differences of cryopreservation methods caused different regrowth rate of cryopreserved (+LN) explants of both cultivars. Regrowth rates after immersion of shoot tips in LN were significantly affected by the duration of loading and dehydration solutions application. For the ‘Bartlett’ cultivar, the highest regrowth percentage was 95.6% after applying LS for 40 min (LS-40) and PVS3 for 90 min (PVS3-90) by method #5. It was 95.4% after exposing LS-50 and PVS3-60 by method #7. These two methods did not show a significant difference in regrowth rate. For the ‘BaeYun No.3’ cultivar, the highest regrowth percentage was obtained at 68.9% after applying LS-40 and PVS3-90 by method #5. The application of LS-50 and PVS3-120 decreased the regrowth rate to 18.4%. Extending the exposure duration from 90 min to 120 min of PVS3 resulted in a decrease in regrowth (Table 1). Chen et al. (2011) have reported that the exposure time of dehydration solution (PVS) is important because it determines the degree of cell dehydration and the amount of cryoprotectants permeating the cells.
Researchers in previous studies have reported a significant role of the regrowth medium in the survival and regrowth of cryopreserved explants after cryopreservation in various plant species (Kim et al., 2012; Wang et al., 2017). We checked the regrowth efficiency of cryopreserved explants using regrowth medium and MS as control medium without PGRs. After cryopreserved steps, shoot tips were transferred to the regrowth medium. As shown in Fig. 4, survival explants can be observed following 2 weeks. Different reaction of the explants exhibited green (Fig. 4A) or black shoot tips (Fig. 4D), indicating that surviving or necrosis of explants. The maximum number of regrowth explants was observed after 8 weeks on regrowth medium (Fig. 4B), while on hormone-free MS medium, it exhibited 0% regrowth (Fig. 4D). To consider genetic resources that have been successfully cryopreserved, a minimum recovery of 40% was determined as the baseline for cryogenic storage of clonally propagated plants (Reed, 2001). Both cultivars had viability levels greater than 40% in this study. The regrowth rate was 95.6% for ‘Bartlett’ cultivar and 68.9% for ‘BaeYun No.3’ cultivar. Various cryopreservation methods have been established for long-term storage of pear genetic resources. However, the most commonly applied approaches, PVS2-based droplet-vitrification, utilize dimethyl sulfoxide (DMSO), which is known for its toxicity and potential to induce DNA methylation in plant/animal cells (Kopnick et al., 2018). Hence, we have developed a cryopreservation method for pear genetic resources using a DMSO free cryoprotectant, PVS3.

Fig. 4.
Development of regrowth plants after cryopreservation by droplet-vitrification using apical shoot tips of pear (Pyrus spp.). (A), green explants on regrowth medium two weeks after cryopreservation. (B), regrowth shoots on regrowth medium 8 weeks after cryopreservation. (C), recovery of regrowth seedling transferred on hormone-free MS medium from regrowth medium. (D), necrosis of explants on hormone-free MS medium 8 weeks after thawing.
In summary, apical shoot tips excised from in vitro grown shoot cultures were chosen as explants for cryopreservation by droplet-vitrification. We finally determined that the most effective method (#5) by droplet-vitrification in the present study involved the following procedures: utilizing apical shoot tips only, preculturing with MS + 0.3 M sucrose for 31 hours and 0.7 M sucrose for 17 hours, loading with LS (35% of PVS3, C4) for 40 min, followed by dehydration using PVS3 (50% glycerol + 50% sucrose) treatment for 90 min and unloading with MS + 0.8 M sucrose for 40 min. Furthermore, we decided to use a regrowth medium consisting of MS with 3% sucrose, 1.0 ㎎/L BA, and 0.1 ㎎/L GA3 to enhance the efficiency of regrowth in cryopreserved explants. Cryopreservation method developed in this study showed a high regrowth rate for European pear (P. communis, 95.6%) and Asian pear (P. pyrifolia, 68.9%). Therefore, this result suggests that the cryopreservation protocol could be widely used as an optimal method for long-term storage of Pyrus germplasm. Optimization of cryopreservation protocols for pear germplasm will also contribute to the development of optimized cryopreservation protocols for other woody plants.




