Introduction
Materials and Methods
Cultivation of plant seedlings
Treatment of growth inhibitors
Comparison of growth by treatment method and treatment concentration of growth inhibitors
Statistical analysis
Results and Discussion
Effects of diniconazole treatment on plant height and hypocotyl length
Effects of diniconazole treatment on leaf length & width and leaf area
Effects of diniconazole treatment on SPAD values
Comparison of effect of the foliage treatment and the irrigation treatment at seedling shipment
Introduction
Crop cultivation begins with seedling production, and healthy seedlings are also important in smart farms system. Plug seedlings for seedling production can be planted in a larger number in the same area than conventional seedlings production, and the seedlings can be uniformly planted and standardized seedlings can be produced on a planned basis throughout the year. However, because of a small cell of the planted plug, it is easy to over grow due to factors, such as an increase in temperature of daytime and night time or a decrease in net photosynthesis during high temperature in summer, rainy season, or in winter with lack of light (Lee et al., 2024; Yun et al., 2007). Until now, hardware parts, such as materials and sowing systems for raising seedlings have developed. However, the importance of software parts, such as environmental control technology (Boursianis et al., 2020; Lakhiaret al., 2018) during seedlings production, is relatively weak, making it difficult to satisfy the quality and economic feasibility of raising seedlings.
The control of growth and development in seedlings is very important in improving seedling quality and reducing management costs (Lee and Kim, 1999; Suh and Chung, 1986). As a method of controlling plant growth that improves seedling quality, various kinds of the methods have reported, such as the method of inducing ethylene generation (Biddington, 1986; Erwin, 1992) and the method of preventing over-growth applying the DIF principle (Lim et al., 1997; Park et al., 1996), the method of controlling irrigation ratio (Kim et al., 1998; Shin, 1998), the method of using salt (Zhang et al., 2003), the method by gamma-radiation (Bae, 1999), the treatment method by light quality after sunset (Zhang et al., 2003), and the method of preventing over-growth using a growth regulator (Gilbert, 1990; Lieberth, 1990). And triazole-based pesticides, which are disinfectants, are mainly used as plant growth inhibitor (Bae, 1999).
As a growth inhibitor, the triazole component has been widely used in flowering and growth control in flowering plants rather than in vegetable crops. On the other hand, the use of triazole-based pesticides, such as paclabutrazole, uniconazole, and diniconazole has been reported in vegetable crops (Gibertz, 1992; Suh and Chung, 1986; Sung et al., 2004; Yun et al., 2007; Zhang et al., 2003). The triazole component is known to inhibit plant growth by inhibiting particulate oxidation of kaurene catalyzed by cytochrome P450 oxidase and laurene oxidase (Deepthi, 2016; Eum et al., 2011; Luster and Miller, 1993). The diniconazole component is used for the purpose of preventing over-growth of seedlings due to high cultivation-density of crops, high temperature and humidity, and lack of light in rasing seedlings (Zhang et al., 2003). Studies on the growth inhibitory effects of triazole have been conducted on various crops (Kim et al., 2016; Sun et al., 2009; Sung et al., 2004; Yun et al., 2007). It was found that there was little residual pesticide after 40 days of treatment for cabbage seedlings and 24 days after treatment for red pepper seedlings (Sun et al., 2002; Sung et al., 2004). And in gourd vegetables, it was found that even in low concentration treatment of 5 ㎎/L, growth inhibition effect can be expected according to rapid absorption and transition (Bae, 1999). In particular, diniconazole, a triazole family, is effective in inhibiting the growth of cucumbers (Sun et al., 2010; Sun et al., 2009), gourds (Kim et al., 1998) and tomatoes (Choi et al., 2001; Sun et al., 2009; Yun et al., 2007), suppression of the growth of highland summer-cabbage (Shin and Jeong, 2002), and suppression of the bolting of spring cabbage (Seong et al., 2003), Moreover, it is widely used as a substitute for expensive growth regulators due to its low unit price.
When using growth inhibitors, the quality of seedlings varies depending on the type of crop, treatment timing, and treatment concentration (Sun et al., 2010; Yun et al., 2007) during the raising seedling. Careful reviewing crops and environmental condition are needed to minimize phytotoxicity of the seedling, because sensitivity of plants by the concentration is high (Shin and Jeong, 2002; Venkatramesh and Croteau, 1989; Zhang et al., 2003). As a commercially available diniconazole, the ‘Binnari’ product (diniconazole 5%), which is recently used in the field, is disclosed for growth inhibition only on cabbage (KCPA, 2014). So when it applied to other crops, it has a problem that violates Positive List-System (PLS). On the other hand, it is necessary to prepare a proper use plan for ‘Binnari’ because it is used to minimize over-growth seedlings or reduce management costs in poor environmental conditions, such as excessive moisture and lack of light. In the current use of ‘Binnari’, growth is mainly suppressed through foliar spraying treatment, but the growth inhibition effect is halved during foliar spraying treatment at high temperatures, and there is a risk of phytotoxicity when repeatedly spraying at high concenztrations and short periods (Shin and Jeong, 2002; Venkatramesh and Croteau, 1989; Zhang et al., 2003). Therefore, it is necessary to investigate the irrigation method to solve the points that does not have the risk of phytotoxicity and maintains the persistence of the growth inhibiting effect after the inhibitor treatment in the process of raising seedlings.
In general, the irrigation method in the nursery includes the head-irrigation method that supplies water from the top of the seedling and the bottom-irrigation method that supplies water to the rhizosphere (Um et al., 2009). The bottom irrigation method can save more than 50% of fertilizer compared to head irrigation because it supplies nutrients and moisture to the rhizosphere without applying physical stimulation to the plant (Tan et al., 2021; Zhang et al., 2022). But most domestic nurseries do not adopt this method, and most of them adopt overhead irrigation (Kang et al., 2023). It is necessary to study a method of maintaining persistence by absorbing diluted diniconazole from leaves and roots by employing this irrigation method. Thus, this study was carried out to search for the effect of diniconazole, a growth inhibitor, in the cultivation of melon, and the growth inhibitory effect according to the treatment method and treatment concentration to obtain basic data on healthy raising seedlings that can be used in the production site, such as raising seedling field and self-cultivation farms.
Materials and Methods
Cultivation of plant seedlings
This study was repeated three times from April 2023 to October 2023 at the Raising Seedling Center at Gokseong Agricultural Cooperative located in Daepyeong-ri, Gokseong-gun, Jeollanam-do, South Korea.
Nursery media were used ‘Luxury Gold’, and artificial mediums for commercial grafting (Seoul Bio Co., Ltd., Korea) and plastic ports (Bumnong Co., Ltd., 50 districts) were used for raising seedlings. Germination was managed at 28℃ and humidity at 95-100% in the germination room by using a sowing machine (Smart Sowing System, Helperovotech, Korea), and then the germinated seeds were moved to nursery.
Environmental management of the nursery was managed by maintaining a ventilation temperature of 20℃ to 26℃, a daytime temperature of 20℃ to 28℃, and a heating temperature of at least 18℃ using an automatic temperature controller (Magma, GreenCs Co., Korea). In order to maintain the heating temperature, air was heated by circulating hot water (60℃) through an electric heater warmer and a hot water pipe. Overhead watering with nutrient components was performed once every two days in summer and once every four to five days in winter according to the existing method of the nursery.
Treatment of growth inhibitors
The concentration of foliar spraying treatment was set to 5 levels (0 ㎎/L, 5 ㎎/L, 10 ㎎/L, 20 ㎎/L and 37.5 ㎎/L) by referring to previous research cases (Bae, 1999). And since there are no prior studies, the irrigation treatment was conducted at the concentration of 0 ㎎/L to 10 ㎎/L in the nursery as a preliminary experiment. As a result of experiments on mini-cucumber (Ministop), spashi-cucumber (Joongbok-Samchuck), tomato (Pink star) and lettuce (Jinchung-mat), the treatment concentration was set to 5 ㎎/L or less in this experiment. Because more than 6 ㎎/L showed severe dwarfing and was not suitable for healthy raising seedling.
Comparison of growth by treatment method and treatment concentration of growth inhibitors
Plant materials were melon ‘Ottugi (LuckyJongmyo)’ variety, and after treating growth inhibitors (5%, Dobang- Agro, Korea) by concentration and treatment method below, the difference in seedling quality was investigated.
Foliar spraying treatment was performed at a total of 5 levels at 0 ㎎/L, 5 ㎎/L, 10 ㎎/L, 20 ㎎/L and 37.5 ㎎/L concentrations, and the treatment was performed three times on the 5 days after germination (May 12, 2023), the 10 days after germination (May 17), and the 15 days after germination (May 22).
Irrigation treatment was treated at a total of 4 levels at 0 ㎎/L, 1.25 ㎎/L, 2.5 ㎎/L and 5 ㎎/L concentrations, and on the 5 days after germination (May 12), 2L per each tray was irrigated once with overhead irrigation method.
Growth surveys were conducted at 5-day intervals after treatment with diniconazole, and final surveys were made at the right time of shipment with seedlings. The survey items were plant height, hypocotyl length, leaf length, leaf width, leaf area and SPAD value. The growth factors except leaf width and leaf areas were measured by tape measure and Vernier calipers (Mitutoyo, Japan). The leaf area was investigated by an estimate using a regression equation for the leaf length and leaf width measurements, which was calculated as leaf length × leaf width × 0.246 (Seo, 2016). The SPAD value was measured three times in the center of the first leaf and the second leaf using a SPAD meter (SPAD-502Plus, Konica Minolta, Japan).
Statistical analysis
Statistical analysis of the data obtained in the study was executed using the SAS program (V.9.4 Cary, NC, USA) to test the significance of the treatment section at the 5% significance level through Duncan’s multiple range test (DMRT).
Results and Discussion
Effects of diniconazole treatment on plant height and hypocotyl length
In order to understand the effect of the treatment method and treatment concentration of diniconazole on seedling quality during the process of raising-seedling in melon, on the 5 days, 10 days and 15 days after germination, 5 ㎎/L, 10 ㎎/L, 20 ㎎/L and 37.5 ㎎/L of diniconazole were treated in the leaf areas, and then growth of the seedlings were investigated on the 5 days, 10 days, 15 days and 20 days after diniconazole treatment. Moreover, on the 5 days after germination, diniconazole was treated at concentrations of 1.25 ㎎/L, 2.5 ㎎/L, and 5 ㎎/L, and the growth surveys were conducted at 5-day intervals after diniconazole treatment.
As a result of foliage treatment of diconazole, as time passed, as the treatment concentration increased compared to the non-treatment, the growth of plant height (Fig. 1) and hypocotyl length (Fig. 2) was suppressed. In particular, in the 37.5 ㎎/L treatment, which is the maximum concentration of foliage treatment, the plant height and the hypocotyl length showed the largest distinction in the single treatment compared to the non-treatment. At the time of seedling shipment, 20 days after germination (Table 1), the growth inhibition was recovered somewhat. But the plant type was not practically suitable for the seedling shipment. Especially, the distinction was the largest in the plant height and the hypocotyl length in the maximum concentration (37.5 ㎎/L) of the foliage treatment compared to the untreated control. These results showed a similar trend to the report of Seonget al. (2003) that even in spring cabbage, when a concentration of diniconazole 35 ㎎/L or more are treated, plants are excessively distorted or nodule due to germination suppression occurs in artificial medium.
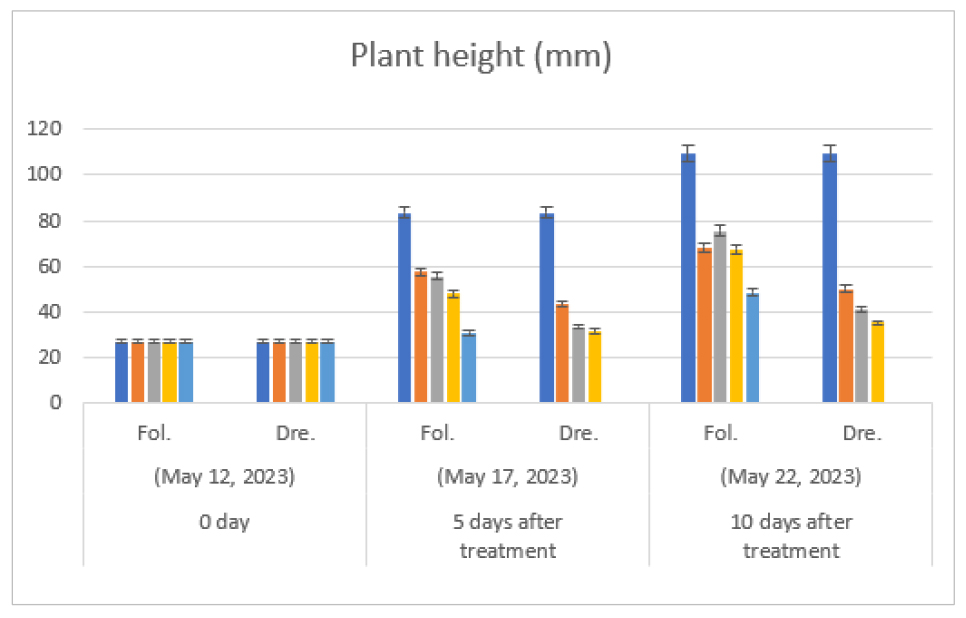
Fig. 1.
Effect of foliage treatment and irrigation treatment with drenching of diniconazole (㎎/L) on plant height (㎜) of melon (Cucumis melo L. cv. Ottugi) seedlings. Seeding date: May 7, 2023, Fol.: Foliage treatment, Dre.: Irrigation treatment with drenching. Fol: Diniconazole was treated with 0, 5 ㎎/L, 10 ㎎/L, 20 ㎎/L and 37.5 ㎎/L (from left to right) on leaf parts of seedling. Dre: 0, 1.25 ㎎/L, 2.5 ㎎/L and 5 ㎎/L (from left to right) on the surface of soil, respectively.
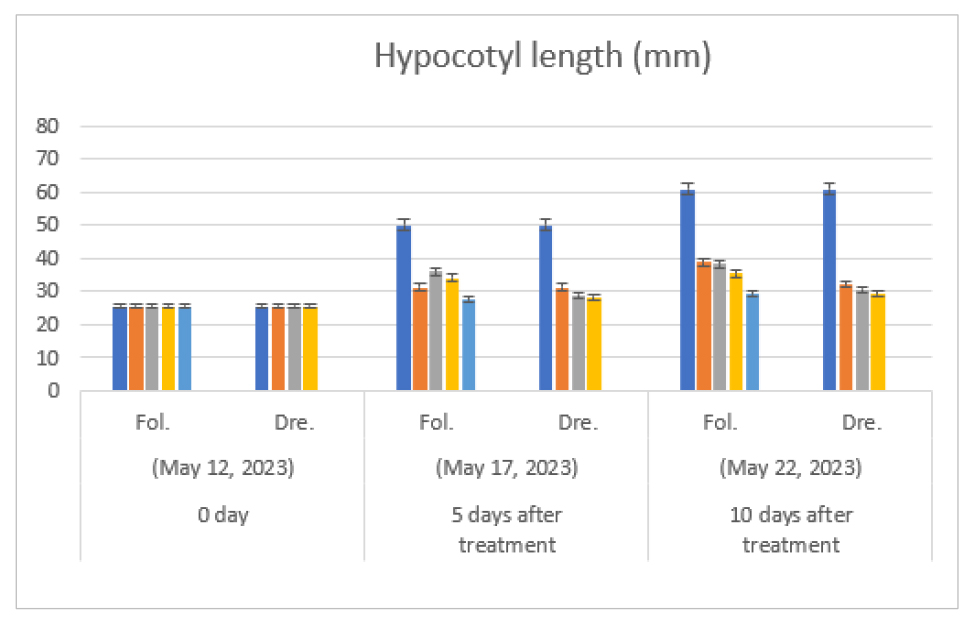
Fig. 2.
Effect of foliage treatment and irrigation treatment with drenching of diniconazole (㎎/L) on hypocotyl length (㎜) of melon (Cucumis melo L. cv. Ottugi). Seeding date: May 7, 2023, Fol.: Foliage treatment, Dre.: Irrigation treatment with drenching. Fol: Diniconazole was treated with 0, 5 ㎎/L, 10 ㎎/L, 20 ㎎/L and 37.5 ㎎/L (from left to right) on leaf parts of seedling. Dre: 0, 1.25 ㎎/L, 2.5 ㎎/L and 5 ㎎/L (from left to right) on the surface of soil, respectively.
During the irrigation treatment with drenching of diniconazole, the higher the treatment concentration of diniconazole, the more plant height was suppressed on the ground (Fig. 1, Table 1). In particular, the lower drainage field was strongly suppressed from the low concentration (1.25 ㎎/L) to the high concentration (5 ㎎/L), resulting in a distinct suppression in hypocotyl length (Fig. 2, Table 1). On the other hand, the irrigation treatment showed stable growth in the primary and lower drainage fields due to a continuous inhibitory effect compared to the foliage treatment, but the plant showed strong inhibition in the high concentration (5 ㎎/L) treatment, and it was judged to be unsuitable for seedling shipment.
The inhibitory effect of diniconazole on hypocotyl length was similar to other plant seedlings (Shin and Jeong, 2002; Sun, 2004). When shipping at nurseries nationwide, the target plant height of melon plug seedling is around 15 ㎝. This is the same as the targeted plant height of cucumbers seedlings (Sun et al., 2010). If the plant height is more than 15 ㎝, the packaging work with seedlings is not adequate, and the settlement of the seedlings on main field may be delayed due to the over-growth of the seedlings. Thus, it should be managed and shipped without over-growth of the seedlings. In this study, it was possible to inhibit the hypocotyl elongation of seedlings stably by the drenching treatment with diniconazole.
Table 1.
Comparison of growth pattern between foliage treatment and irrigation treatment with drenching of diniconazole just before the seedling shipment day in melon (Cucumis melo L. cv. Ottugi)z.
|
Diniconazole (㎎/L) |
Plant height (㎜) |
Hypocotyl length (㎜) |
Leaf length (㎜) |
Leaf width (㎜) |
Leaf area (㎝2) | SPAD |
No. of leaves | ||||||||
| Fol.y | Dre. | Fol.y | Dre.x | Fol.y | Dre.x | Fol.y | Dre.x | Fol.y | Dre.x | Fol.y | Dre.x | Fol.y | Dre.x | Fol.y | Dre.x |
| 0 | 0 | 112.50±1.80a | 112.50±1.80a | 70.67±0.76a | 70.67±0.76a | 70.53± 0.70a | 70.53± 0.70a | 75.53± 1.03a | 75.53± 1.03a | 131.07±30.73a | 131.07±30.73a | 42.80± 2.33d | 44.30± 4.05b | 3 | 3 |
| 5 | 1.25 | 79.07±0.90b | 53.00±1.67b | 51.43±0.93b | 32.60±1.04b | 66.57± 0.80b | 50.43± 0.81b | 70.2± 0.92b | 55.07± 1.60b | 114.96±28.85b | 68.33± 30.14b | 45.5± 0.56c | 49.67± 0.35a | 3 | 3 |
| 10 | 2.5 | 77.83±1.65b | 42.83±0.91c | 48.87±1.36c | 31.90± 0.56bc | 63.63± 1.46c | 48.2± 0.75c | 66.03± 0.65c | 51.13± 1.03c | 103.38±33.42c | 60.64± 21.33c | 47.27± 0.86bc | 50.47± 0.15a | 3 | 3 |
| 20 | 5.0 | 70.90±0.66c | 37.43±0.68d | 43.50±0.89d | 30.53± 0.95c | 58.03± 0.68d | 43.33± 1.04d | 62.4± 0.66d | 49.47± 1.12c | 89.09± 19.36d | 52.75± 24.74d | 49.43± 0.64ab | 51.47± 0.64a | 3 | 3 |
| 37.5 | -w | 50.63±1.55d | - | 34.07±1.45e | - | 42.63± 0.47e | - | 49.47± 1.08e | - | 51.88± 16.17d | - | 50.90± 0.85a | - | 3 | - |
Effects of diniconazole treatment on leaf length & width and leaf area
During the foliage treatment, a greater inhibitory effect on growth was shown at high concentrations in leaf-length, and the inhibitory effect was greater in the second main leaf than in the first main leaf (Fig. 3-A). The leaf width was lower than that of the leaf-length, and the inhibitory effect by the diniconazole treatment was similar in the first and second main leaves (Fig. 3-B). The leaf area decreased as the treatment concentration of diniconazole in both the first and second main leaves increased (Fig. 3-C).
During the irrigation treatment with drenching of diniconazole, leaf length, leaf width, and leaf area showed a clear inhibitory effect at all concentrations compared to the foliage treatment (Fig. 3, Table 1), and showed a continuous inhibitory affect and stable growth compared to the foliage treatment. In this way, melon showed more severe dwarfism in the irrigation treatment than that of the foliage treatment with high concentrations (more than 5 ㎎/L), making it unsuitable for transplanting into main crop field. In addition, as a result of the preliminary experiment, male flowers blossomed early, resulting in additional work efforts due to the removal of male flowers when shipped (data not shown). In order to over come the early flowering, it is suggested that the irrigation treatment at a concentration of less than 5 ㎎/L is needed when the leaf length of the main leaf is developed for approximately 1 ㎝ to 2 ㎝. On the other hand, the growth inhibitory effect of diniconazole treatment first appears as leaf-length elongation inhibition from one week after treatment at high concentrations in this study, Seong et al. (2003) also, reported stem elongation inhibition in other plants. And it is necessary to treat diniconazole into the seedlings by referring to the mechanism of action of diniconazole.
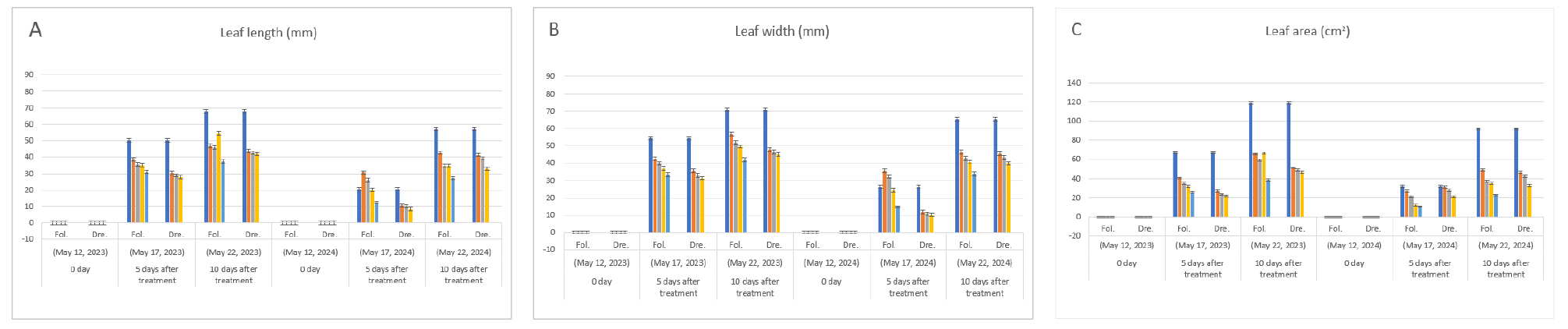
Fig. 3.
Effect of foliage treatment and irrigation treatment with drenching of diniconazole (㎎/L) on leaf length (A), leaf width (B) and Leaf area (C) of melon (Cucumis melo L. cv. Ottugi). Seeding date: May 7, 2023, Fol.: Foliage treatment, Dre.: Irrigation treatment with drenching. Graphs on left side show data from 1st true leaf and on the right show from 2nd true leaf, respectively. Fol: Diniconazole was treated with 0, 5 ㎎/L, 10 ㎎/L, 20 ㎎/L and 37.5 ㎎/L (from left to right) on leaf parts of seedling. Dre: 0, 1.25 ㎎/L, 2.5 ㎎/L and 5 ㎎/L (from left to right) on the surface of soil, respectively.
Effects of diniconazole treatment on SPAD values
The higher the concentration of diniconazole in foliage treatment and irrigation treatment with drenching of melon, the higher the concentration of SPAD (Fig. 4). The SPAD value during the drenching treatment increased as the treatment concentration of diniconazole increased and tended to increase steadily until the seedling shipment (Fig. 4, Table 1).
In this regard, the higher the treatment concentration of diniconazole, the higher the SPAD value in tomato, cucumber and hot pepper (Zhang et al., 2003). Moreover, Khalil (1995) said that when triazole was treated, the number of cells remained unchanged, but as the leaf area decreased, the number of chlorophyll per unit area became denser. Thus, the concentration of chlorophyll was relatively higher than that of the untreated zone.
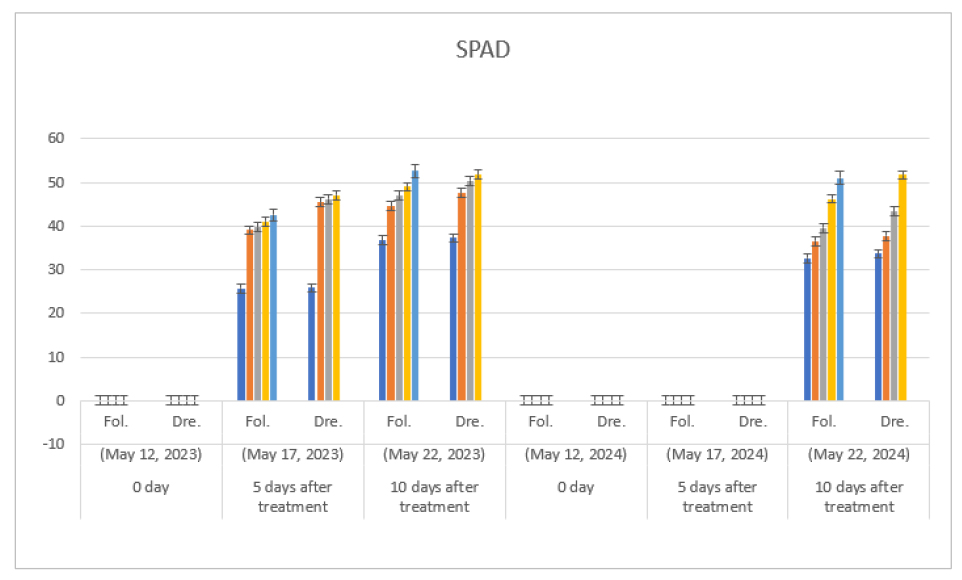
Fig. 4.
Effect of foliage treatment and irrigation treatment with drenching of diniconazole (㎎/L) on SPAD of melon (Cucumis melo L. cv. Ottugi). Seeding date: May 7, 2023; Fol.: Foliage treatment, Dre.: Irrigation treatment with drenching. Graphs on left side show data from 1st true leaf and on the right show from 2nd true leaf, respectively. Fol: Diniconazole was treated with 0, 5 ㎎/L, 10 ㎎/L, 20 ㎎/L and 37.5 ㎎/L (from left to right) on leaf parts of seedling. Dre: 0, 1.25 ㎎/L, 2.5 ㎎/L and 5 ㎎/L (from left to right) on the surface of soil, respectively.
Comparison of effect of the foliage treatment and the irrigation treatment at seedling shipment
Especially, the effect of diniconazole on inhibition of seedling growth was highlighted at the step of seedling shipment day. Comparing the effects of diniconazole on the foliage treatment and the irrigation treatment with drenching in melon ‘Ottugi’ cultivar, the higher the treatment concentration in both methods, the better the effect of some kind of inhibition (Table 1). Based on the time of shipment of seedlings, the appropriate concentrations of the foliage treatment and the drenching treatment were 20 ㎎/L of the foliage treatment and 1.25 ㎎/L of the drenching treatment, respectively. And the dwarfness was severe at the concentration of 37.5 ㎎/L of foliage treatment and 5 ㎎/L of the drenching treatment, making it impossible to shipment for plug seedlings. As a result, even at a concentration 16 times lower than that of the foliage treatment, the growth inhibitory effect of the seedling continued and strong in 1.25 ㎎/L of the drenching treatment.
The persistence of the inhibitory effect was maintained for about 15 days after once treatment in the irrigation treatment with drenching, and the inhibitory effect was maintained for 5 days by the foliage treatment. During the production of melon seedlings, the inhibitory effect of growth by diniconazole treatment was cleared for both the leaf treatment and the irrigation treatment with drenching treatment. However, the irrigation treatment, which showed the persistent effect of inhibiting growth, was more effective in the production of healthy seedlings. For this reason, the foliage treatment should be treated more than three times at an appropriate concentration, and even if only one is omitted, over-grown seedlings are occurred. On the otherhand, the irrigation treatment with drenching was more effective in seedling production because the inhibitory effect due to the treatment of the growth inhibitor continued until the time of shipment after treatment once in this study.
As a result of the above, it was similar to the report of Fisher et al. (1996) that the persistence and the inhibitory effect of plant growth inhibitors in the foliage treatment were generally proportional to the concentration of the growth inhibitors initially treated. In the case of the irrigation treatment with drenching, it is reported that the higher concentration and longer treatment times of pacrobutrazole into seeds, the slower the growth of seedlings in marigolds, geraniums, tomatoes (Pasian and Bennett, 2001; Shin and Jeong, 2002), cucumbers (Cho et al., 2002) and peppers (Shin and Jeong, 2002). Although, there are no cases in melons so far, it was reported that there was the same optimal concentration of diniconazole treatment in the same gourd crops, such as cucumber and zucchini (Jeong, 2024). Various varieties of melons must be investigated in the further study with diniconazole treatments for production of healthy plug seedlings. On the other hand, Dicks and Charles Edwards (1973) reported that determining stem elongation after harvest in edible chrysanthemum (Chrysanthemum morifolium) treated with daminozide, a growth inhibitor, is related to the concentration of daminozide remaining in the stem. Thus, the persistent effect on growth inhibition during drenching treatment of melon is seemed to have an effect on maintaining the persistence of growth inhibition by remaining growth inhibitors in the stem which is absorbed by the root in this study.




