Introduction
Materials and Methods
Experimental material
Experimental material extraction
Fraction preparation
Isolation of complex saponin
Cell culture and calcium mobilization control
Isolation of the basilar artery in the rabbit brain
Blood vessel tension measurement
Statistical analysis
Results
Discussion
Introduction
Hypertension, a chronic degenerative disease, is a risk factor for cardiovascular disease, affecting 12.5% of adults worldwide. In Korea, approximately 30 out of 100 adults (30%) over the age of 30 suffer from this disease, making the total annual national social medical expenses approximately KRW 5.678 trillion. Although the exact cause of hypertension is unknown, complex factors such as race, family history, salt intake, insulin resistance, aging, and alcohol consumption are all implicated (Hong et al., 2022). The Renin–Angiotensin System (RAS) plays an important hormonal role in regulating blood pressure. When RAS is activated, renin breaks down angiotensin into angiotensin I, which in turn acts as an angiotensin for vasoconstriction by the angiotensin I-converting enzyme (ACE). It is then converted to angiotensin II, which plays a role in causing reabsorption of sodium ions, increased excretion of potassium ions, production of aldosterone that increases blood flow, vasoconstriction, and increase in blood pressure. Currently known ACE inhibitors include ramipril, captopril, enarapil, risinopril, fosinoril, and spirapril, which are widely used as antihypertensive agents (Reeves et al., 1991; Tuladhar et al., 1996; Tytgat et al., 1997). However, because the above compounds are easily decomposed into pharmaceutical dosage forms, they have poor stability and act on other cells to cause side effects, such as general weakness, cough, vomiting, headache, and anorexia. Therefore, developing ACE inhibitors with excellent inhibitory activity and stability and antihypertensive agents with different pharmacological mechanisms is highly desirable (Garcia-Colunga et al., 1997; Jagadesh and Subhash, 1998; Ni and Miledi, 1997; Park and Park, 2020).
Platycodon grandiflorum and Glycyrrhiza uralensis contain several bioactive compounds, such as saponin, oleanolic acid, and flavone. P. grandiflorum and G. uralensis have traditionally been used to treat disorders related to blood pressure, diabetes, and counteracting poison; they also have antinociceptive and antiinflammatory properties. However, the validity of complex saponin’s vasodilatory effect has not been scientifically investigated. Recently, Koreans are easily exposed to various lifestyle-related and chronic degenerative diseases due to westernized dietary changes, so the demand for health- functional food is increasing (Bak et al., 2009; Kim et al., 2020; Shin, 2000; Yang et al., 2019). As natural compounds contained in various plant resources are being investigated for use as functional materials, analyzing the physiologically active ingredients of various horticultural crops grown solely for ornamental purposes for use in various fields is necessary.
Previous reports on P. grandiflorum and G. uralensis are representative research results, such as compound isolation and general physiological activity studies. Preclinical studies on major diseases, such as hypertension, remain incomplete. Therefore, in this study, an in vivo assay was performed to explore the vasodilatory effect of complex saponin isolated from P. grandiflorum and G. uralensis mixed ethanol extract, and the oriental mixed formulations potential as a functional material with high safety and few side effects was suggested (Busch et al., 2000; Leung et al., 1996; Pitt et al., 1994; Pacher et al., 2001; Villazon et al., 2002).
Materials and Methods
Experimental material
Whole plants of P. grandiflorum and G. uralensis were collected from Mt. Sebong, Pyeongchang County, Gang-won Province, Korea, in November 2019 and were identified by Dr. J. N. Lee. A voucher specimen (NIHA.P-15-1) was deposited in the herbarium of the Highland Agriculture Research Institute, Pyeongchang, Korea.
Experimental material extraction
Whole plants of P. grandiflorum and G. uralensis were pulverized to 2 ㎏ at a mixing ratio (6:4) and then chilled with ethanol solvent at room temperature for 6 months. The mixing ratio was referred to the oriental medical manufacturing method (Donguibogam). After filtering the ethanol extract with qualitative filter paper, the filtrate was concentrated using a rotary vacuum concentrator (Tokyo Rikakikai Co., Tokyo, Japan) to obtain an ethanol extract. Table 1 lists the measurement results.
Table 1.
The yield of P. grandiflorum and G. uralensis Ethanol extract
| Sample | Ethanol extractz | |
| Weight (g) | Yield (%) | |
| P. grandiflorum and G. uralensis | 220 | 11 |
Fraction preparation
After suspending 220 g of P. grandiflorum and G. uralensis ethanol extract in 0.8 L of distilled water, n-Hexane (1 L × 3), chloroform (1 L × 3), ethylacetate (1 L × 3), and buthylalchol were examined in the order of solvents from polar to nonpolar. Table 2 lists the measurement results after eluting with (1 L × 3) and vacuum concentration of each fraction.
Table 2.
The yield of P. grandiflorum and G. uralensis organic solvent fractions
| Solvent fractionz | P. grandiflorum and G. uralensis | |
| Weight (g) | Yield (%) | |
| n-Hexane | 28.2 | 14.1 |
| Chloroform | 39.3 | 19.7 |
| Ethylacetate | 47.1 | 23.6 |
| Buthylalchol | 49.2 | 24.6 |
Isolation of complex saponin
Dried substances of P. grandiflorum and G. uralensis were cut and extracted three times with ethanol under reflux and evaporated to obtain a viscous mass. ethanol ext. was dried in the vacuous. P. grandiflorum and G. uralensis mixed ethanol extracts were fractionated with buthylalchol . The complex saponin was isolated from the buthylalchol fraction using the Diaion HP-20 resin adsorption method. The complex saponin (4.1 g) isolated using this process was used as a sample for the vascular relaxation experiment.
Cell culture and calcium mobilization control
The vascular smooth muscle cell line, A7r5, was cultured at 37℃ in a 5% CO2-95% air incubator with DMEM culture medium supplemented with 10% FBS, penicillin 10 unit/mL, streptomycin 100 ㎍/mL, and 2 mM L-glutamine. The monolayer-cultured cells in the culture flask were washed twice with basal medium and then treated with 2.5% trypsin solution to detach the cells. The suspended cells were then neutralized with a serum medium and centrifuged, after which the cell pellet was washed three times with the cell culture medium. Furthermore, a hemocytometer was used to measure the number of cells, which was adjusted to realize a concentration of 5 × 104 cells/mL , and 200 μL of each cell was placed in a 96-well plate (Corning, dark-clear bottom) and incubated for 24 h. Cells cultured in the 96-well plate were treated with Fura-2/AM (molecular probe) at a concentration of 5 μM, maintained in a CO2-incubator at 37℃ for 1 h, and washed three times with Ca2+-free PSS. After adding 190 μL of PSS containing 1.5 mM Ca2+ to each well, kylenol, which was to be tested, was treated at a concentration of 10 μg/mL, and the assay plate was inserted into Flexstation 3. A 5-fold concentrated K+ solution was inserted into the compound plate to maintain a final concentration of 50 mM K+ when added. After K+ was automatically added using Soft Max Pro 5.1, the intensity of fluorescence was measured (Cauvin et al., 1983; Huang, 1996; Karaki et al., 1997; Li et al., 1993; Park et al., 2008; Velasco et al., 1997).
Isolation of the basilar artery in the rabbit brain
The basilar artery used in this study was isolated using the surgical method. First, white rabbits weighing 2–2.5 ㎏ were anesthetized by inhaling enflurane, an anesthetic for animals, and then the basilar artery at the bottom of the brain was excised while the skull was cut. The excised basilar artery was immersed in a Ca2+-free Tyrode solution saturated with 95% O2 and 5% CO2 to remove the surrounding connective tissue and fat. The Ca2+-free Tyrode solution (in μM) used for blood vessel separation comprising 137 NaCl, 5.4 KCl, 1 MgCl2, 23.8 NaHCO3, 5.5 glucose, and sucrose was used to adjust the osmotic pressure to 300 mOsm/㎏ H2O (Cauvin et al., 1983; Huang, 1996; Karaki et al., 1997; Li et al., 1993; Park et al., 2008; Velasco et al., 1997).
Blood vessel tension measurement
The separated blood vessels were tested by storing them in a Ca2+-free Tyrode solution saturated with 95% O2 and 5% CO2. First, the basilar artery was cut to a size of 5 mm and fixed to the end of the force transducer using a chromium ring and to the clasp ring at the bottom of the organ bath maintained at 37℃. Isometric contraction was measured while keeping the inside of the organ bath continuously saturated with 95% O2 and 5% CO2 by measuring the contractile force when both ends were fixed. By replacing the blood vessel in the normal Tyrode state with a Tyrode containing a high concentration of potassium (50 mM) ions, the membrane potential was depolarized to -20 mV according to the Nernst equation, and the passage through which the ions passed was closed. By activating the inactivated L-type calcium channel, the calcium concentration in the vascular smooth muscle cells was increased to induce contraction, and the contraction and relaxation were repeated three times. The reactivity of the endothelial cells of the basilar arteries used in this experiment was confirmed by checking whether the contractile force of blood vessels decreased when the Tyrode solution containing a high concentration of potassium ion was used to induce contraction and then treated with acetylcholine (1 μM). After stabilizing the basilar artery for 10 min with Tyrode’s solution containing a high concentration of potassium ions, kylenol was treated for about 10 min at different concentrations to confirm the relaxation effect. Endothelin- induced vasoconstriction was reduced by treatment with 3 nM of endothelin-1. The Tyrode solution (in μM) used in the vasoconstriction and relaxation experiment comprised 137 NaCl, 5.4 KCl, 1.5 CaCl2, 1 MgCl2, 23.8 NaHCO3, and 5.5 glucose, and the Tyrode solution (in μM) containing high concentration of potassium ions was 92.4, comprising NaCl, 50 KCl, 1.5 CaCl2, 1 MgCl2, 23.8 NaHCO3, and 5.5 glucose. The osmotic pressure was maintained at 300 mOsm/kg H2O, and the pH was adjusted to 7.4 by saturation with 95% O2 and 5% CO2 (Cauvin et al., 1983; Huang, 1996; Karaki et al., 1997; Li et al., 1993; Park et al., 2008; Velasco et al., 1997).
Statistical analysis
The experimental results were expressed as mean ± SE (Standard Error), and the change in vasoconstriction was expressed as the actual contraction size and the percentage and mean ± SE of the maximum contraction induced by the contraction substances (potassium and endothelin). The significance of each experimental result was determined using the Student’s t-test.
Results
To check whether the calcium mobilization force in vascular smooth muscle cells, which is a major factor in vasoconstriction, can be controlled, Fura-2 calcium ion-specific fluorescent dye was loaded onto A7r5 cells and then excited at 340/380 nm wavelength. As shown in Table 3, the emission wavelength intensity was 1.85 ± 0.1 μM on average when a high potassium concentration was used as a control group, and treatment with complex saponin isolated from P. grandiflorum and G. uralensis (30 ㎍/mL) yielded 1.62 ± 0.1 μM. An inhibitory efficacy of μM was observed. As a result, vasoconstriction inhibitory efficacy could be predicted by a high potassium concentration in studying vasotension control.
Table 3.
Tension regulation in the vascular smooth muscle cell line
| Sample |
Regulation of potassium-induced calcium mobilizing powerz (F340/380 ratio, μM) |
|
Complex saponin isolated from P. grandiflorum and G. uralensis (30 ㎍/mL) | 1.62 (± 0.1) |
|
Negative control (Only cell) | 1.85 (± 0.1) |
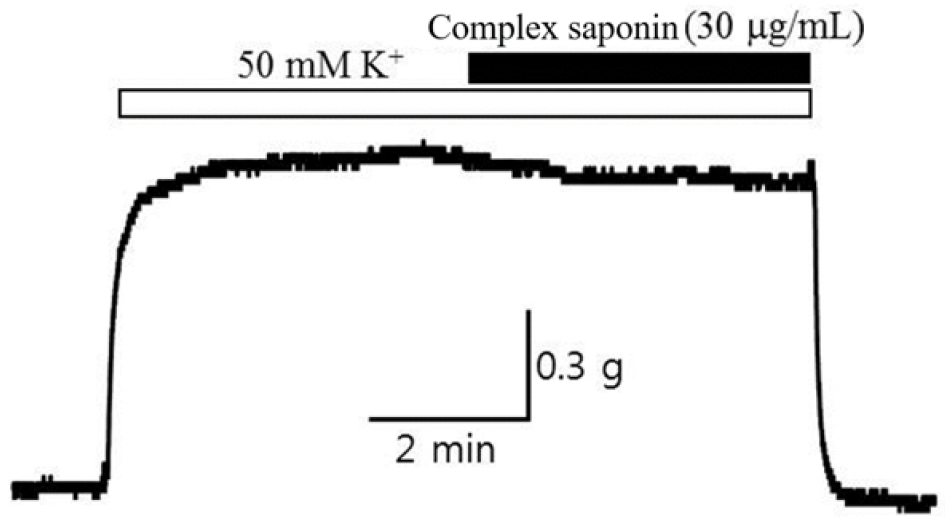
Basic blood vessel tension or blood vessel tension altered by certain conditions manifests as vascular smooth muscle contraction and relaxation. In particular, blood vessel contraction is a membrane voltage-dependent L-type calcium. This is related to the increase in cytoplasmic calcium ions by the inflow of calcium ions through the channel (Voltage- dependent Ca2+ channel). When an electrical stimulus (e.g., excitation wave transmission from the dominant nerve in the blood vessel) stimulates the vascular smooth muscle, this muscle is electrically depolarized and the L-type calcium channel opened by the membrane potential remains open. It is then activated, and calcium ions flow from the outside to the inside depending on the concentration difference of calcium ions, resulting in vascular smooth muscle contraction and increased tension in blood vessels. Therefore, in this study, the relaxation effect of complex saponin isolated from P. grandiflorum and G. uralensis (30 ㎍/mL) treatment was investigated on the vasoconstriction that occurs when depolarization is artificially induced using a high potassium concentration. First, a high potassium concentration Tyrode solution was applied to the rabbit basilar artery, and when vasoconstriction was stabilized after approximately 10 min, complex saponin isolated from P. grandiflorum and G. uralensis was treated, and the degree of relaxation of constricted blood vessels was measured for 10–15 min, from which improved vasodilatory efficacy was observed (Table 4, Fig. 1).
Table 4.
Vasodilatory potency of complex saponin isolated from P. grandiflorum and G. uralensis on potassium-induced constriction of the rabbit basilar artery
| Sample | Vasodilatory efficacyz (EC50)y |
| Basilar arteryx (%) | |
|
Complex saponin isolated from P. grandiflorum and G. uralensis (30 ㎍/mL) | 15.7 (± 4.0) |
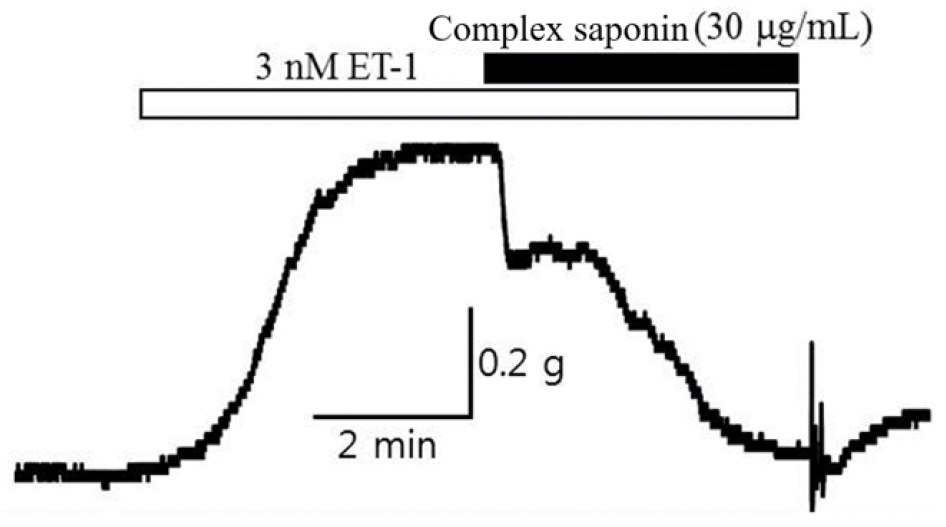
Accordingly, it showed the strongest vasoconstriction effect in the human body, and the relaxation effect on vasoconstriction induced by endothelin, a major target of diseases such as diabetes, hypertension, and renal failure, was verified. After constricting the basilar artery of the brain with 3 nM endothelin, a relaxation concentration (EC50) was observed when complex saponin isolated from P. grandiflorum and G. uralensis 30 ㎍/mL was treated. As shown in Table 5 and Fig. 2, an effective vasodilatory efficacy of 92.4 ± 3.1% or more was observed. In the case of endothelin, complex saponin isolated from P. grandiflorum and G. uralensis can be a good candidate for an endothelin receptor inhibitor. The blood concentration of endothelin increases more than 100 times in patients with diabetes, leading to hypertension, renal failure, foot necrosis, and peripheral circulation disorders.
Table 5.
Vasodilatory potency of complex saponin isolated from P. grandiflorum and G. uralensis on endothelin-induced constriction of the rabbit basilar artery
| Sample | Vasodilatory efficacyz (EC50)y |
| Basilar arteryx (%) | |
|
Complex saponin isolated from P. grandiflorum and G. uralensis (30 ㎍/mL) | 92.4 (± 3.1) |
Discussion
Hypertension is defined as an adult systolic blood pressure of 140 mmHg or higher or diastolic blood pressure of 90 mmHg or higher (Kim and Kim, 2018). However, patients with high blood pressure rarely show symptoms, so it is not diagnosed until blood pressure is measured. In oriental medicine, P. grandiflorum and G. uralensis were mainly used as prescription agents for hypertension treatment(Ji, 2016; Nam, 2020) so P. grandiflorum and G. uralensis were used to evaluate the relaxation effect of the rabbit basilar artery forced to contract with endothelin. In this study, the vasodilatory effect was evaluated after isolating complex saponin from P. grandiflorum and G. uralensis. Endothelin, known as the most powerful vasoconstrictor in the human body, acts on endothelin receptor subtype A (ETAR) present in the vascular smooth muscle cell membrane and receptor subtype B2 (ETB2R) present in the vascular endothelial cell membrane to increase vascular tone. It acts as a modulator to reduce tension (Lucchelli et al., 1999). In general, endothelin peptides increase blood levels in inflammation, diabetes, and cardiovascular diseases, and an abnormal hyperactivity of the endothelin system causes renal diseases, such as chronic renal failure and glomerulosclerosis, and respiratory diseases, such as idiopathic pulmonary fibrosis and chronic obstructive pulmonary disease. It causes diseases such as diabetic neuropathy, retinopathy, hand and foot necrosis, and cancer diseases such as prostate and colon, which are important points of interest in mechanical and metabolic diseases (Lavoie et al., 1997; Pancrazio et al., 1998). In this study, the concentration (EC50) of complex saponin isolated from P. grandiflorum and G. uralensis, which inhibited endothelin-induced cerebral basilar artery constriction, was observed. Therefore, structural and functional derivation studies should explore complex saponins isolated from P. grandiflorum and G. uralensis as new materials for regulating endothelin activity to develop health-functional food or pharmaceutical materials.