Introduction
Materials and Methods
Animals and Reagents
Preparation of RF
Induction of Colitis by DSS and Experimental Design
Disease Activity Index (DAI)
PGE2 Measurement
Western blot analysis
Statistical analysis
Results
Effects of RF on weight loss in DSS-induced colitis in mice
Effects of RF on colon shortening in DSS-induced colitis in mice
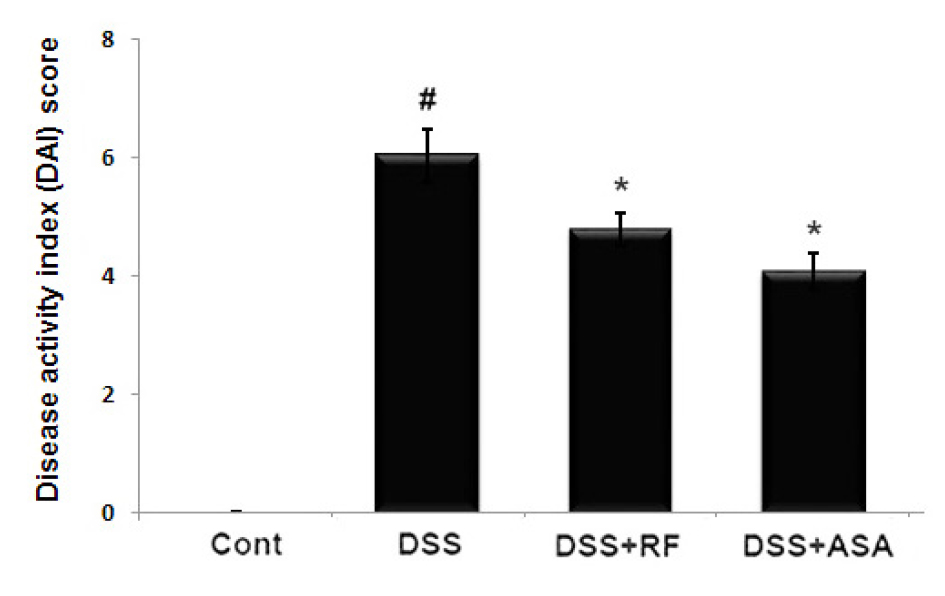
Effects of RF on the DAI in DSS-induced colitis in mice
Effects of RF on COX-2 and PGE2 levels in DSS-induced colitis in mice
Effects of RF on NF-κB activation in DSS-induced colitis in mice
Discussion
Introduction
Ulcerative colitis (UC) is a typical inflammatory intestinal disease belong to the category of inflammatory bowel diseases (IBD). It is characterized by diarrhea, bloody stool, colonic mucosal ulceration, and in severe cases, systemic symptoms (Yashiro, 2014). The pathogenesis of UC is believed to result from the interaction of genetic, immune, and environmental factors (Baumgart and Carding, 2007). The current drugs for UC include glucocorticosteroids, sulfasalazine, 5-aminosalicylic acid, and others (Domenech, 2006; Sandborn and Targan, 2002). However, these available therapies cause adverse drug reaction for long-term usage. Thus, traditional herbal medicines have gained attention in developing the treatment of UC.
Cyclooxygenase (COX) generate various prostaglandins (PGs) and two COX isoenzymes have been identified: COX-1 and COX-2. Especially, COX-2, an enzyme induced by various stimuli, generates PGs associated with the mediation of inflammation (Ferrer et al., 2019; Tsuge et al., 2019). Recent studies reported that the COX-2 and PGE2 levels were increased in the inflamed mucosa of patients with UC (Roberts et al., 2001). Therefore, therapeutic agents that inhibit the levels of COX-2 and PGE2 may effectively ameliorate intestinal inflammation.
Nuclear factor (NF)-κB is a transcription factor and stimulates the expression of many genes involved in the inflammatory process (Tak and Firestein, 2001). It was reported that NF-κB was overexpressed in intestinal epithelial cells of UC patients (Rogler et al., 1998). These findings suggest that NF-κB is involved in controlling the levels of inflammatory mediator, which is involved in the mucosal tissue damage that occurs in UC. Consequently, agents that effectively regulate the NF-κB pathway would be beneficial for the treatment of UC.
Rubi Fructus (RF), the fruit of Rubus coreanus Miquel (Jung et al., 2021), has used in traditional herbal medicine for the treatment of inflammation, obesity, hepatic steatosis and liver lipotoxicity (He et al., 2015; Jeong et al., 2015; Nam et al., 2014). However, there is no information on whether RF regulates intestinal inflammation, including UC.
In the present study, to provide experimental evidence that RF might be a useful therapeutic in UC, we assessed the beneficial effects and mechanism of RF in dextran sulfate sodium (DSS)-induced colitis in mouse model. The specific goals were: (1) to explore the effect of RF on the clinical signs of colitis such as weight loss, colon shortening, diarrhea, and bloody stool and (2) investigate the regulatory effect of RF on the levels of COX-2, and PGE2 as well as the activation of NF-κB in colon tissues.
Materials and Methods
Animals and Reagents
BALB/c mice (six-week-old males) were purchased from the Hyochang Science (Daegu, Korea). The mice were maintained in a SPF environment for at least one week to allow them to adapt to the environmental changes. Animals were kept under a 12-h light/dark cycle at room temperature (24 ± 2℃) and humidity (56 ± 10%). DSS (mol wt; 36,000-50,000) was purchased from MP Biomedicals (Solon, OH, USA). Specific antibodies against histone, β-actin, NF-κB, and COX-2 were purchased from Santa Cruz Biotechnology (Santa Cruz, Dallas, USA). Enhanced chemiluminesence (ECL) and nuclear extraction reagent kits were obtained from Pierce Thermo Scientific (Rockford, IL, USA). Aminosalicylic acid (ASA) and other reagents were procured from Sigma-Aldrich (St. Louis, MO, USA).
Preparation of RF
The fruit of RF (100 g) was chopped using a blender and extracted with 1 L of 70% aqueous ethanol solution for 24 h and then concentrated under a vacuum. The extract obtained was filtered (Whatman, GD/X, USA), concentrated in a water bath under vacuum, frozen and lyophilized lyophilized (Sunileyela, EYELA-FDU-1200, Japan) to yield the ethanol extract (yield: 11.9%).
Induction of Colitis by DSS and Experimental Design
Colitis was induced in the mice by administering 5% (w/v) DSS drinking water ad libitum for seven days. The mice (n=7) were checked daily for body weight loss, stool consistency, and the presence of gross bleeding. Mice were randomized into 4 groups (n=7/ group): vehicle, DSS, DSS plus RF treatment (100 ㎎/㎏) and DSS plus ASA (100 ㎎/㎏). RF and ASA (positive control) were orally administered once a day during DSS treatment and after which the mice were sacrificed.
Disease Activity Index (DAI)
Intestinal disease activity was assessed based on weight loss, the presence of diarrhea accompanied by blood and mucus, and colonic shortening (Hendrickson et al., 2002). The severity of colitis was calculated by DAI assessment, a scoring system which includes three parts as described by Wirtz et al. (2017): body weight loss (0-4), degree of intestinal bleeding (0-4) and stool consistency (0-4). The DAI values were examined by three investigators blinded to the protocol.
PGE2 Measurement
The PGE2 concentration in DSS-induced colon tissue was measured by a PGE2 colorimetric assay kit according to the manufacturer’s directions (Enzo Life Science , NY, USA).
Western blot analysis
For Western blots, the section of distal colon (5-10 ㎜) were homogenized in PRO-PREP protein extraction buffer (Intron Biotechnology, Seoul, Korea). Nuclear extracts were prepared using nuclear extraction reagent kit. After protein quantification using BCA, the samples were mixed with a sample buffer, boiled and then separated by electrophoresis on a 10% denaturing gel. After electrophoresis, the protein was transferred on to the membrane. The membranes were reacted with the primary antibodies, and after washing with 0.1% PBST, incubated with secondary antibodies for 2 h. The antibody-specific proteins were visualized by an ECL detection system (Pierce Thermo Scientific, IL, USA).
Statistical analysis
The results are presented as the mean ± S.E.M and each experiment was performed at least- three times. The statistical data were analyzed using independent t-tests and ANOVA with a Tukey post hoc test. P value of < 0.05 was considered significant.
Results
Effects of RF on weight loss in DSS-induced colitis in mice
The DSS-induced colitis experimental model has a phenotype similar to that of human UC (Ramakers et al., 2007). The clinical symptoms of colitis (body weight loss, colon shortening, bloody stool) induced by DSS were observed. First, the improvement in the weight loss by RF treatment of DSS-induced colitis mice was evaluated. As shown in Fig. 1, the mice treated with DSS significantly lost a weight compared to the control group. However, the RF groups showed a significant attenuation of weight loss caused by DSS. ASA was used as a positive control in the present study.
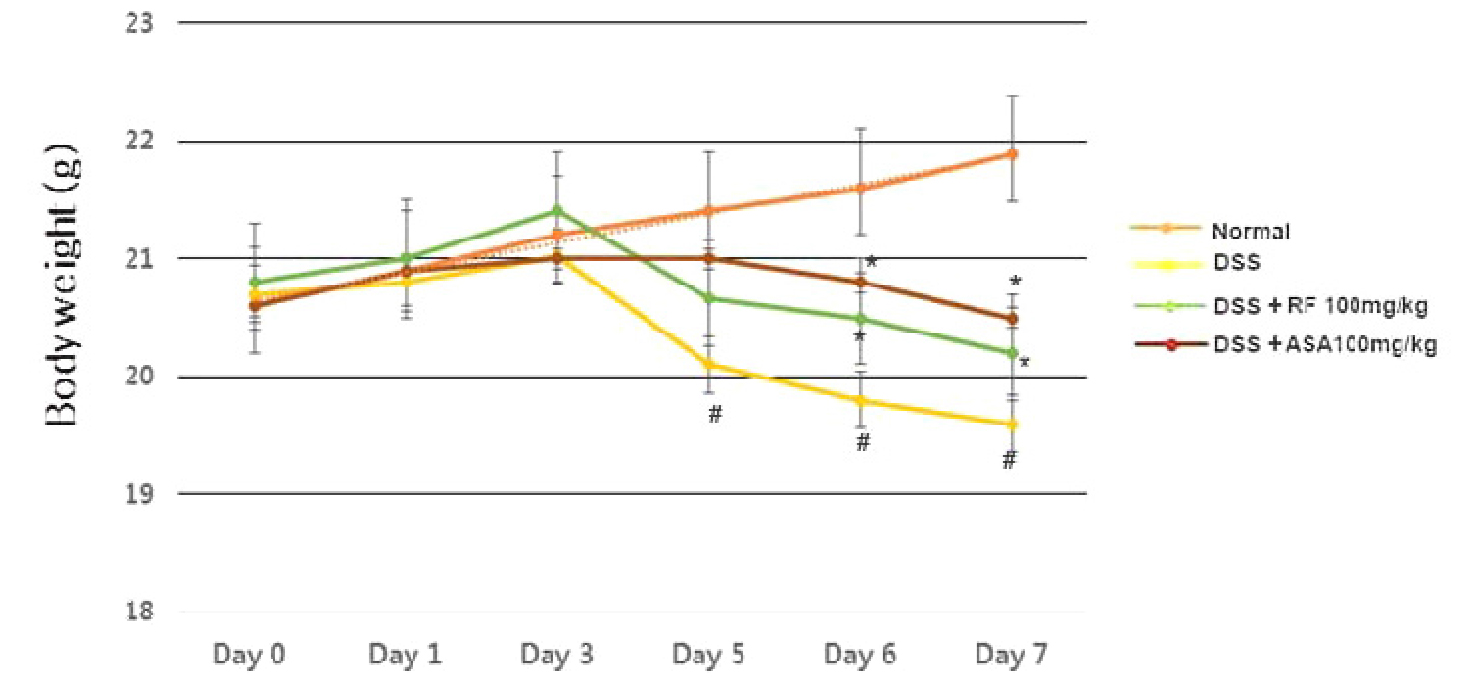
Fig. 1
Effect of RF on DSS-induced the body weight loss in mice. Experimental colitis was induced by administering a 5% DSS in the drinking water for 7days. RF (100 ㎎/㎏) and ASA (100 ㎎/㎏, the reference drug) were orally administered for over the same period. Body weight of mice was measured at the same time on the experimental days. Data were represented in the mean ±S.E.M. (n = 7) and results were analyzed by Tukey post hoc test (#P < 0.05 versus control and *P< 0.05 versus DSS alone).
Effects of RF on colon shortening in DSS-induced colitis in mice
Colon length measurement has been used as a morphological parameter for the degree of intestinal inflammation (Hendrickson et al., 2002). We investigated the effect of RF on colon length in DSS-induced colitis. Seven days following DSS treatment, we found that the colon length in the DSS-administered mice was significantly shorter (7.80 ± 0.55 cm) than that of the control (4.28 ± 0.31 ㎝). However, RF treatment alleviated the DSS effects on colon shortening (5.31 ± 0.39 ㎝) (Fig. 2A). The relative colon lengths are shown in Fig. 2B.
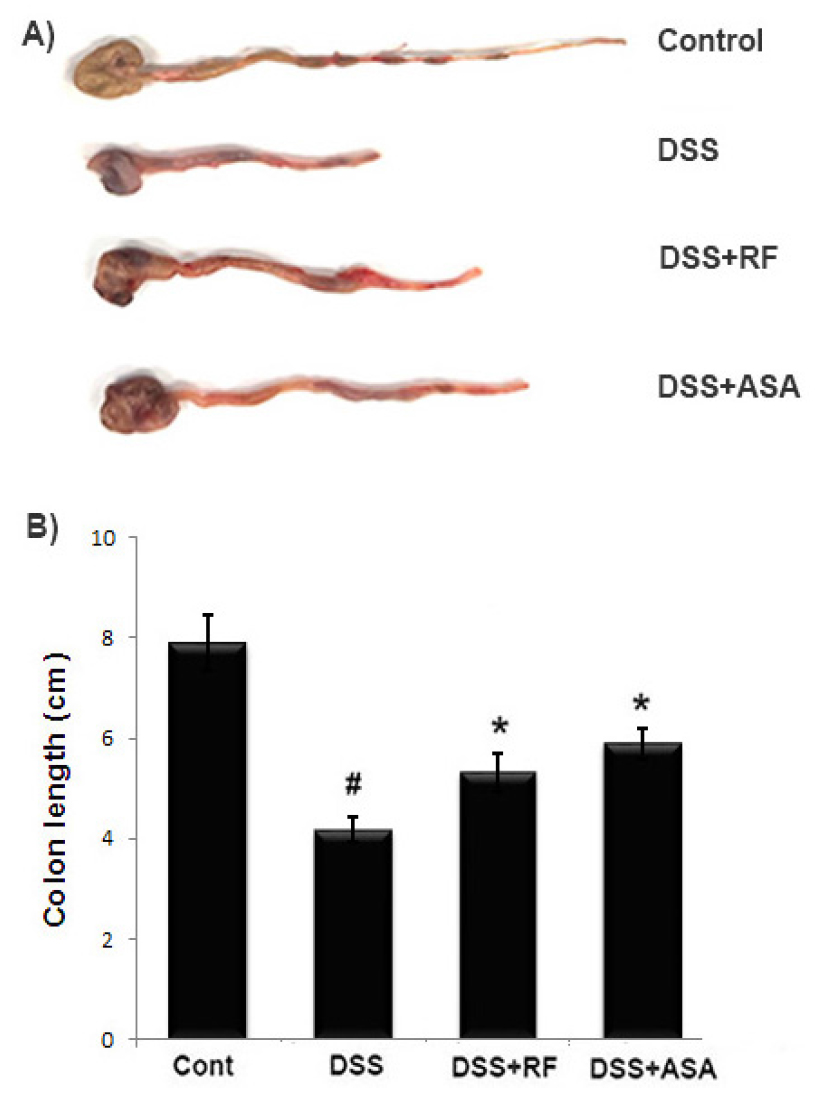
Fig. 2
Effect of RF on DSS-induced the colon length shortening in mice. Experimental colitis was induced by administering a 5% DSS in the drinking water for 7days. RF (100 ㎎/㎏) and ASA (100 ㎎/㎏, the reference drug) were orally administered for over the same period. (A) The colons were removed at day 7 after DSS treatment, and the colon lengths were measured. (B) Relative colon lengths were represented. Data were represented in the mean ±S.E.M. (n = 7) and results were analyzed by Tukey post hoc test (#P < 0.05 versus control and *P< 0.05 versus DSS alone).
Effects of RF on the DAI in DSS-induced colitis in mice
The common feature of the DSS-induced model of colitis is an increase in the DAI (Wirtz et al., 2017). Generally, the DAI was calculated based on body weight loss, diarrhea, and rectal bleeding degree. We evaluated the regulatory effect of RF on the DAI increase in DSS-induced colitis. The results showed that RF administration prevented the increase in DAI scores induced by DSS (Fig. 3).
Effects of RF on COX-2 and PGE2 levels in DSS-induced colitis in mice
As increase in COX-2 and PGE2 levels are associated with the physiological processes of UC (Agoff et al., 2000), we investigated the effect of RF on COX-2 and PGE2 levels in DSS-induced colitis tissue. At the end of the experiment, colon tissues were excised and homogenized. As shown in Fig. 4A, DSS markedly induced COX-2 expression in colon tissue versus the controls. However, the DSS-induced COX-2 expressions were significantly inhibited by RF and ASA treatment. The relative COX-2 levels are shown in Fig. 4B COX-2 catalyzes the biosynthesis of PGE2 Therefore, we evaluated RF to determine whether or not it exerted an effect on PGE2 production. The results showed that the PGE2 levels in colitis tissue were significantly decreased in the RF-treated group (Fig. 4C).
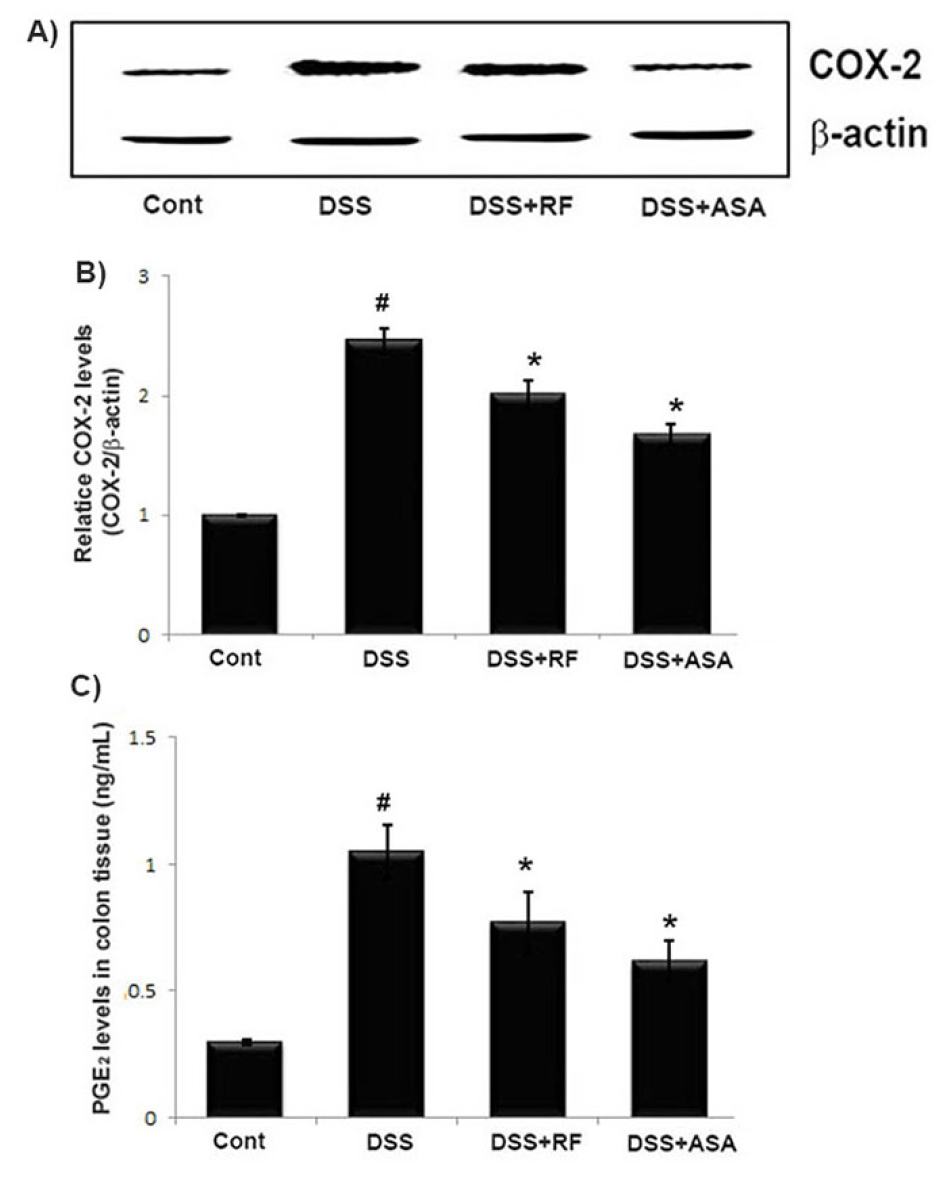
Fig. 4
Effect of RF on the COX-2 and PGE2 levels in DSS-induced colitis tissue. Experimental colitis was induced by administering a 5% DSS in the drinking water for 7days. RF (100 ㎎/㎏) and ASA (100 ㎎/㎏, the reference drug) were orally administered for over the same period. At the end of the experiment, the colon tissues were excised and homogenized. (A) The levels of COX-2 in colonic tissue were assayed by Western blot analysis. (B) The relative COX-2 level was determined using an image analyzer (VILBER Lourmat, France). (C) The amount of PGE2 levels in colonic tissue was measured via immunoassay kits. Data shown are expressed as the means ± S.E.M (#P < 0.05 versus control and *P< 0.05 versus DSS alone).
Effects of RF on NF-κB activation in DSS-induced colitis in mice
Because NF-kB p65 activation is involved in UC (Periasamy et al., 2020), we examined whether RF regulated the activation of NF-kB p65 in DSS-induced colitis tissue. The activation of NF-kB p65 was significantly increased in the colon tissues of DSS-treated mice compared to the controls. However, treatment with RF reduced the activation of NF-kB p65 in these tissues (Fig. 5A). The relative level of NF-kB was measured using an image analyzer (Fig. 5B).
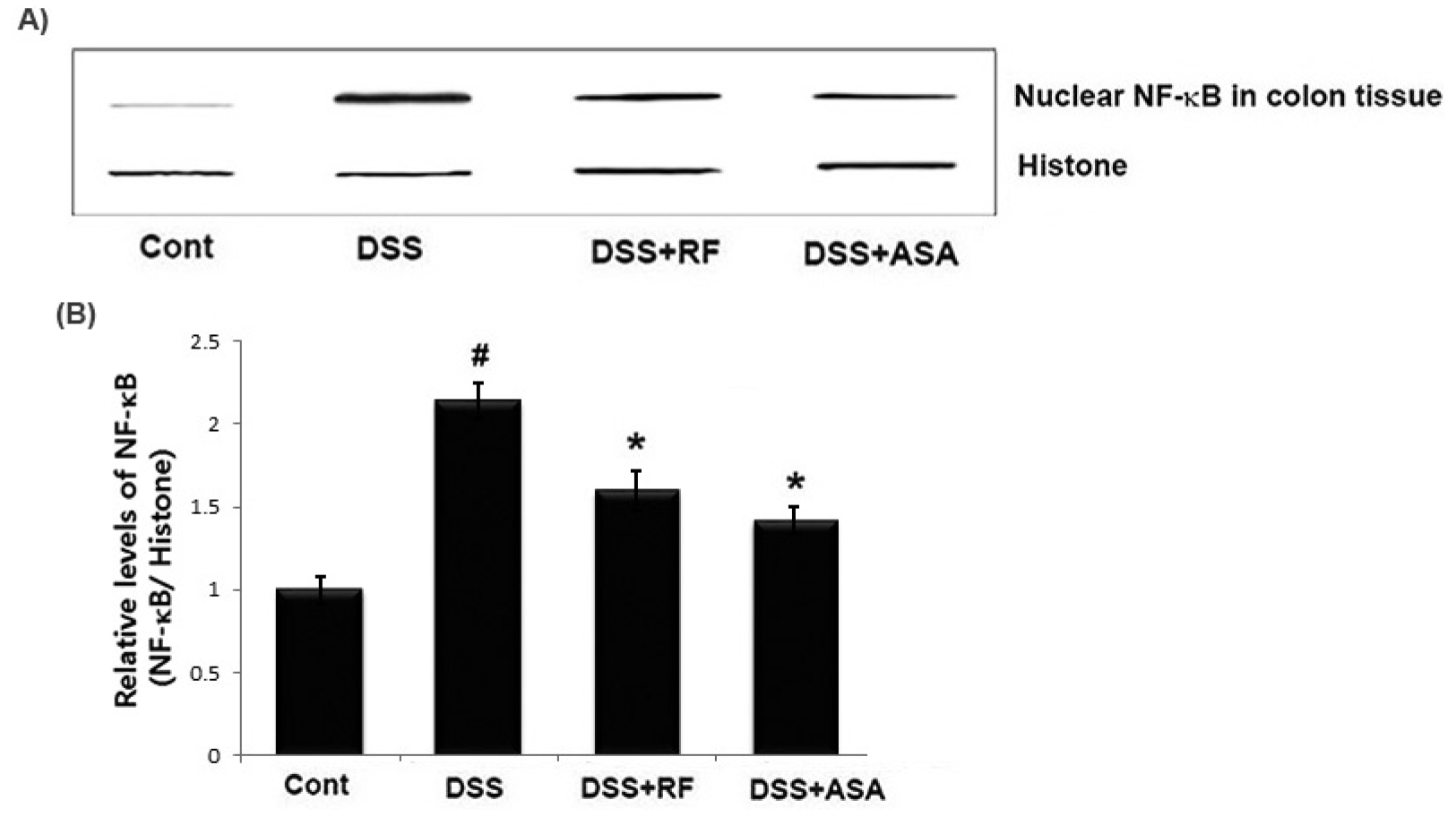
Fig. 5
Effect of RF on the NF-kB activation in in DSS-treated colon tissue. At the End of the experiment, the colon tissues were excised and homogenized. Nuclear extracts in colonic tissue were prepared by nuclear extraction reagent kit. (A) The levels of NF-kB p65 were assayed by Western blot analysis. (B) The relative ratio of NF-kB was calculated using an image analyzer. Data shown are expressed as the means ± S.E.M (#P < 0.05 versus control and *P< 0.05 versus DSS alone).
Discussion
Although traditional herbal medicine is a kind of the alternative treatment options and has been increasingly recognized worldwide, the exact pharmacological mechanisms of most herbs remain limited. In present study, we tried to provide experimental evidence that RF might be a useful therapeutic agent for UC patients. The finding of this study demonstrated that RF suppressed the clinical signs and inflammatory response of colitis provoked by DSS, suggesting the molecular mechanism by which RF ameliorated intestinal inflammation.
UC is characterized by chronic inflammation of the intestinal tract, and mucosal immunity, the intestinal environment, external stimulation, and many other complicated factors are also involved in the pathogenesis of UC (Danese et al., 2004). The current therapeutic drugs for UC include glucocorticosteroids, 5-ASA, sulfasalazine, immunosuppressant, and others (Domenech, 2006; Sandborn and Targan, 2002). However, these drugs cause serious side effects for long-term usage. Recently, the interest in traditional herbal medicines for the treatment of these disorders has increased. Thus, this study investigated the improvement in DSS-induced colitis by RF treatment. The important signs of UC were body weight loss, diarrhea and bloody stools (Ardizzone and Bianchi, 2005; Hudcovic et al., 2001). In this study, we observed that mice treated with DSS showed body weight loss and colon shortening compared to the control group. However, treatment with RE suppressed the weight loss and colon shortening induced by DSS. The DAI values, which was scored using three major clinical symptoms (body weight loss, diarrhea, and rectal bleeding), were exhibited a remarkably higher than that of the control mice. However, these increases were significantly inhibited in groups administered RF. These results suggest that RF exerts anti-colitis activity by regulating the clinical symptoms of colitis caused by DSS.
Accumulated experimental evidence has shown that inflammatory mediators were associated with the pathogenesis of intestinal inflammation including UC (Lin et al., 2018). It was reported that COX-2 and PGE2 levels were elevated in patients with UC and that they played an integral role in UC pathogenesis (Li et al., 2018). Thus, we attempted to investigate the ameliorative effect of RF the COX-2 and PGE2 levels in DSS-induced colitis tissue. The results showed that the levels of COX-2 and PGE2 were increased in DSS-induced colitis tissue compared to those in controls and that treatment with RF prevented the increases in COX-2 and PGE2. From this, we suggest that the anti-inflammatory activity of RF may be associated with the suppression of inflammatory mediators including COX-2 and PGE2 in DSS-induced colitis tissue.
The production of inflammatory mediators is dependent upon the activation of NF-κB in intestinal inflammation (Skupsky et al., 2020). It was reported that NF-kB activation and the subsequent increase in inflammatory mediators is important in UC pathology. Additionally, NF-κB plays a crucial role in the disruption of epithelial barrier function. Therefore, suppression of NF-κB activation has been demonstrated as an anti-inflammatory strategy in treatment of UC (Atreya et al., 2008). In this study, we observed an increase of NF-kB activation in DSS colon tissues compared to the control, and that administration with RF reduced these levels. These results indicated that RF attenuated intestinal inflammation and alleviated the symptoms of UC by blocking the NF-κB activation in DSS-induced colitis. Although we revealed the role of RF treatment in the attenuation of NF-kB activation in colitis, the regulatory effects of RF on other pathways including MAPK-signaling were not clarified. Therefore, other studies must be performed to elucidate the precise mechanism of RF in the inhibition of the intestinal inflammatory response.
In conclusion, our results showed that RF exerted an anti-colitis activity by alleviating the clinical symptoms in DSS-induced colitis in mice. In addition, we suggest that the anti-inflammatory effect and mechanism of RF could be attributed to the suppression of inflammatory mediators (COX-2 and PGE2) and NF-kB activation in colon tissue. These results provide experimental evidence that RF might be a useful therapeutic candidate for treating intestinal inflammatory disorders including UC.