Introduction
Materials and Methods
Plant material and culture establishment
Preparation of plant extract
Estimation of total phenolic content
Estimation of total flavonoid content
Analysis of Antioxidant Activities
Quantification of phenolic compounds by high-pressure liquid chromatography
Statistical analysis
Results
Influence of different media plant regeneration
Influence of strength of the MS medium on plant regeneration
Influence of sucrose concentration supplemented to MS medium on plant regeneration
Estimation of phenolics, flavonoids and antioxidant activities in Lobelia chinensis regenerated plants
HPLC analysis of phenolics in the regenerated plants
Discussion
Introduction
Lobelia chinensis Lour., often known as “Chinese lobelia” is a member of the Campanulaceae family which is a perennial plant found in China, Japan, and Korea. It is an important medicinal herb utilized in Korean, Japanese, and Traditional Chinese Medicine (TCM) systems of medicine. It has been used in TCM to treat edema, snake bites, high fevers brought on by malaria, diarrhea, and jaundice (Li et al., 2016). Several pharmacological effects of Lobelia chinensis have been reported, including anti-inflammatory (Li et al., 2015), antioxidant (Zhang et al., 2018), antiviral (Kuo et al., 2011), anti-obesity (Zhang et al., 2020), anti-tuberculosis (Choi et al., 2016), and anticancer effects (Santosa et al., 1986).
Many secondary metabolites have been identified from Lobelia chinensis, including phenolics, flavonoids, alkaloids, coumarins, terpenoids, and polyacetylenes (Yang et al., 2014). Among these substances, phenolics, and flavonoids are regarded as the primary phytochemicals, which are responsible for biological activities (Jo et al., 2021). Herbal medicine and extracts from Lobelia chinensis are available on the market, but there have been multiple complaints of adulteration in commercial items. Thus, in order to screen actual commercial items, a high-resolution melting technology - DNA barcoding methodology has recently been developed (Sun et al., 2017). Plants grown from natural stands accumulate different amounts of bioactive compounds according to soil type, location, and the impact of biotic and abiotic environments. Large-scale in vitro regeneration of superior clones and the distribution of this biomass to pharmaceutical companies could partially address this problem. However, methods for in vitro propagation of Lobelia chinensis are not available. Therefore, in the current study, we developed an in vitro propagation method for Lobelia chinensis using nodal segments, and we evaluated various parameters affecting the production of biomass and phenolic compounds, such as different medium compositions, medium salt strengths, and sucrose concentration. In addition, we estimated the amount of phenolics, and flavonoids in the regenerated plants and assessed the antioxidant capabilities.
Materials and Methods
Plant material and culture establishment
Stem segments containing one or two nodes and alternate leaves without roots (1.5 ㎝ in length) were collected from stock plants of Lobelia chinensis which were maintained in the greenhouse at Chungbuk National University, Korea. The explants were surface disinfected with 1% (w/v) Bavistin (Bayer, Korea) and then washed for five minutes in 10% (v/v) Tween-20 (Sigma-Aldrich, St. Louis, Mo, USA). Following surface sterilization with 0.1% (w/v) aqueous mercuric chloride (HgCl2) for a period of 2-3 minutes, the explants were placed in 95% alcohol for 20–30 seconds. Then explants were washed in sterile distilled water three times. The explants were then grown in MS (Murashige and Skoog, 1962), B5 (Gamborg et al., 1968), N6 (Chu et al., 1975), SH (Schenk and Hildebrandt, 1972) or Woody plant (McCown and Loyd, 1981) or NLN (Nitsch and Nitsch, 1969) media supplemented with 3% sucrose. Prior to autoclaving 2.4 g L-1 gelrite was added and the pH of the medium was set at 5.7 with 1N HCl or NaOH and then autoclaved for 15 minutes at 15 psi and 121°C. All of the chemicals were of an analytical grade (Duschefa, Harlem, The Netherlands). 15 mL of medium was distributed to 60 ㎜ × 15 ㎜ Petri-dishes. Explants were cultivated horizontally on the medium surface, dishes were sealed with parafilm, and cultures were incubated in a tissue culture room at a temperature of 25°C, with a 16 light/8 dark photoperiod that provided 40 µmol m-2 s-1 of irradiance, cool fluorescent lamps, and 60% relative humidity. Explants were subcultured once in four weeks.
In another set of experiments, cultures were established using quarter, half, full, and double-strength MS media to check the effect of salt strength on the growth performance of explants. In these experiments, a full-strength MS medium served as the control. In one more set of experiments, the MS medium was supplemented with different concentrations of sucrose viz. 1, 3, 5, and 7% (w/v) and medium without sucrose served as control. All the cultures were maintained for four weeks and data on the number of shoots per explant, shoot length (㎝), leaf area (㎟), number of roots, root length (㎝), fresh weight (㎎/explant), and dry weight (㎎/explant) were documented.
Preparation of plant extract
The dried samples were extracted using extracation apparatus (LS-2050-S10, LS-TECH, Korea) with 30 mL 80% ethanol at 80℃ for 1 h and passed through filter paper (Advantec 110 ㎜, Toyo Roshi Kaisha Ltd., Japan). The final volume of the solution was set at 30 mL using 80% (v/v) ethanol.
Estimation of total phenolic content
Total phenolic content was estimated by using the Folin Ciocalteu reagent method (Harborne, 1994). Briefly, a known amount of sample was taken and made up to 3 mL with distilled water, and 0.1 mL of 2 N Folin Ciocalteu reagent was added, followed by incubation for 6 minutes, and then 0.5 mL of 20% Na2CO3 was added to each tube. Tubes were kept in warm water for 30 minutes and the absorbance was read at 760 ㎚ using a UV-visible spectrophotometer. Gallic acid was used as the standard compound.
Estimation of total flavonoid content
The flavonoid content of extracts was analyzed as per the method of Harborne (1994). The extract (0.1 mL) was taken and made up the volume to 3 mL by using distilled water followed by the addition of 0.15 mL of 10% AlCl3 and 2 mL of 1 M NaOH after 5 min of incubation at room temperature. Solutions were vortexed and absorbance was measured at 510 ㎚. Catechin was used as standard.
Analysis of Antioxidant Activities
2,2 Diphenyl 1 picrylhydrazyl (DPPH) radical scavenging assay
Extract (0.1 mL) was added with 1.9 mL of 0.1 mM DPPH solution prepared in ethanol. The tubes were vortexed and incubated in the dark for 15 min. The discoloration of the DPPH solution was measured at 517 ㎚ against ethanol as blank using a UV-visible spectrophotometer. Gallic acid was used as standard and the activity of the extracts was expressed as ㎎ gallic acid equivalent (GAE)/g extract (Munteanu and Apetri, 2021).
2,2’-azino-bis (3-ethybenzothiazoline-6-sulphonic acid) (ABTS) assay
The ABTS solution was prepared by mixing 7 mM of ABTS and 2.45 mM potassium persulfate in a ratio of 1:1 and stored in the dark for 24 h. At the time of analysis, the ABTS solution was diluted with phosphate buffer (pH 7.3) to obtain the value of 0.70 at 732 ㎚. Fifty microliters of the extract were added to 950 microliters of diluted ABTS solution and the mixture was allowed to stay in the dark for 10 min then absorbance was measured at 732 ㎚ using UV-visible spectrophotometry. Antioxidant activity was expressed in percentage i.e., ABTS radical scavenging activity = absorbance of control solution-absorbance of sample solution/ absorbance of control solution × 100 (Munteanu and Apetri, 2021).
Ferric reducing antioxidant power (FRAP) assay
FRAP reagent was prepared by mixing three solutions: a 300 mM acetate buffer (pH 3.6), a 10 mM solution of 2,4,6-tripyridyl-s-triazine (TPTZ) in 40 mM HCl, and a 20 mM FeCl2.6H2O. They were mixed in the ratio of 10:1:1. 0.2 mL of extract was added with 3 mL of FRAP reagent, tubes were vortexed and incubated for 6 min at room temperature, and absorbance was measured at 593 ㎚ using a UV-visible spectrophotometer. Ascorbic acid was used as standard and activity is expressed as ㎎ ascorbic acid equivalent (AAE)/g extract (Munteanu and Apetri, 2021).
Quantification of phenolic compounds by high-pressure liquid chromatography
Extraction and analysis of phenolic compounds were carried out by the procedure of Burin et al. (2011). Fresh plant samples (0.1 g) were extracted in 2.0 mL of 70% methanol containing 2% formic acid and centrifuged at 10,000 g for 10 min at 4℃. The supernatant was filtered through 0.22 µm syringe filters prior to analysis. High-performance liquid chromatography (HPLC) analysis was performed in triplicate using a Shimadzu (Kyoto, Japan) liquid chromatography, equipped with a vacuum degasser (DGU-14A), quaternary pump LC-10AT, UV-vis detector (SPD-10AV) and an injector (Rheodyne) with a 20 µL loop. The CLASS-V software (v. 6.1) was used to control gradient settings, UV-vis, and data acquisition. A C18 reversed-phased column (4.6 ㎜ × 250 ㎜, 5 µm particle size) was used. The mobile phase consisted of acetic acid in filtered Milli-Q water adjusted to ㏗ 2.6 as solvent A and 20% of solution A in 80% acetonitrile as solvent B and the flow rate was 1.2 mL/min. Phenolic compounds were identified by comparing retention times and UV spectra with authentic standards (Sigma-Aldrich, St. Louis, Mo, USA).
Statistical analysis
The results are presented as mean values and standard errors. One-way analysis of variance (ANOVA) was performed to determine significant differences between each treatment. The statistical significance of the differences between mean values was then assessed by Duncan’s multiple range test at p < 0.05. All statistical analyses were performed using the SAS 9.4 software (SAS Institute Inc., Cary, NC, USA).
Results
Influence of different media plant regeneration
In the present study, the influence of various media such as MS, B5, SH, WPM, N6, and NLN was tested on shoot regeneration and accumulation of biomass of Lobelia chinensis, and the data are presented in Table 1. The shoot segments that were cultured on the media involved in the regeneration of axillary shoots at the nodal region and a single shoot developed from each node in the first week, multiple shoots were developed from the second week onwards and leaves differentiated at nodes alternatively, and simultaneously developed roots beneath the nodal region. Profuse shoot regeneration and growth were evident at the end of four weeks. Lobelia is a decumbent plant and thus branches initially grow horizontally on the medium (Fig. 1A). At the end of four weeks the fresh weight, dry weight, number of shoots, shoot length, leaf area, number of roots, and root length were calculated with explants cultured on each media. Growth and accumulation of biomass were highest with MS medium, especially the number of shoots (4.5/explant), shoot length (4.9 ㎝), and leaf area (20.0 ㎠) (Table 1; Fig. 1A). Whereas, the number of roots per explant and root length was significant with the explants cultivated on N6 medium (Table 1 and Fig. 1A). The biomass i.e. fresh weight (405 ㎎/explant) and dry weight (35 ㎎/explant) of the plantlets were optimal with MS medium.
Table 1.
Effect of different basal media on plant regeneration and growth of Lobelia chinensis plants after 4 weeks of culture.
| Medium |
FWz (㎎/explant) |
DWy (㎎/explant) |
No of shoots/explant |
Shoot length (㎝) |
Leaf area (㎟) |
No of roots/explant |
Root length (㎝) |
| MSx | 405aw | 35.0aw | 4.5aw | 4.9aw | 20.0bw | 9.6abw | 2.0bw |
| B5x | 324b | 29.1a | 3.3b | 4.6ab | 19.0b | 10.5ab | 2.1b |
| SHx | 397a | 34.8a | 2.8bc | 4.4ab | 22.0a | 9.4ab | 1.7b |
| WPMx | 354b | 30.4a | 3.4b | 4.0b | 27.3a | 9.6ab | 2.0b |
| N6x | 342b | 30.1a | 2.4c | 3.6b | 17.0c | 11.4a | 2.5a |
| NLNx | 292c | 26.4b | 3.2b | 4.2ab | 18.0c | 8.3b | 1.9b |
Influence of strength of the MS medium on plant regeneration
Data on the growth and biomass accumulation of Lobelia chinensis is presented in Table 2 and Fig. 1B. Of the various strengths of the MS medium tested, the full-strength MS medium was found suitable, and the fresh weight (252.0 ㎎/explant), and dry weight (22.6 ㎎/explant) accumulation. The number of shoots (5.0/explant), shoot length (5.9 ㎝), leaf area (17.8 ㎟), and the number of roots (6.3/explant), and root length (1.6 ㎝) were also optimum with plantlets grown on full strength MS medium. The salt strength of the MS medium is another factor that influences the regeneration potential of medicinal plants. In Lobelia chinensis full-strength MS medium was found good for the regeneration and accumulation of biomass.
Table 2.
Effect of different strength MS medium on plant regeneration and growth of L. chinensis plants after 4 weeks of culture.
Influence of sucrose concentration supplemented to MS medium on plant regeneration
In the present study, various levels of sucrose (1, 3, 5, and 7%) were tested on the growth of Lobelia chinensis, and results are documented in Table 3 and Fig. 1C. The 3% sucrose was found to be optimal for the growth of Lobelia chinensis plants, 215.0 ㎎/explant fresh weight, 21.0 ㎎/explant dry weight, 5.0 shoots per explant, 5.0 ㎝ shoot length, 14.5 ㎟ leaf area, 5.6 roots per explant, and 2.7 ㎝ root length was achieved. The increment of sucrose beyond 3% was not beneficial for the growth of the plants.
Table 3.
Effect of different sucrose concentrations supplemented to MS medium on plant regeneration and growth of L. chinensis plants after 4 weeks of culture.
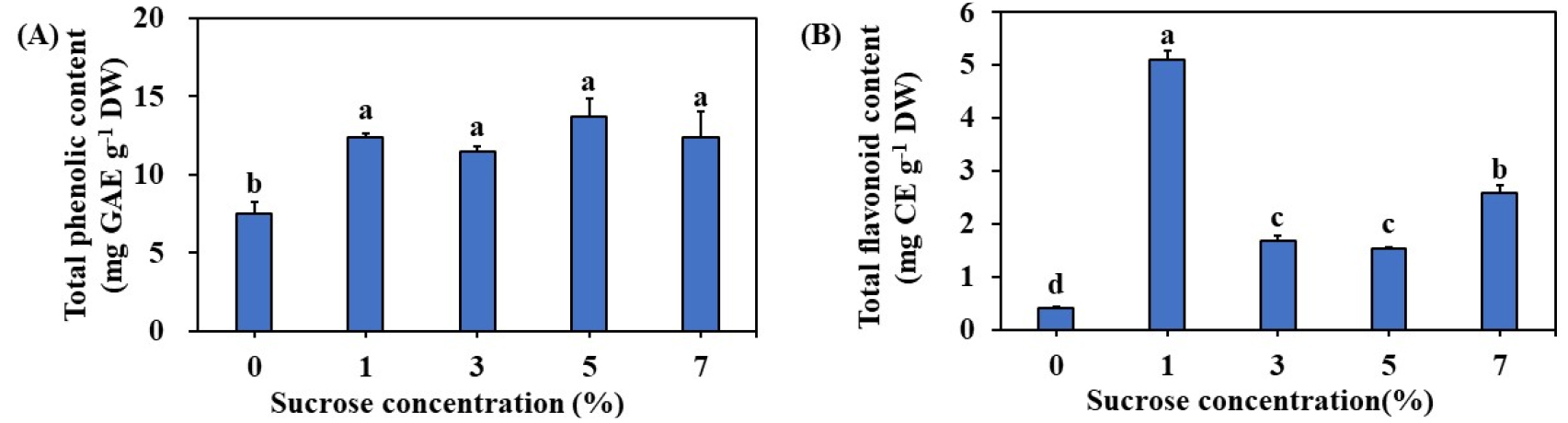
Estimation of phenolics, flavonoids and antioxidant activities in Lobelia chinensis regenerated plants
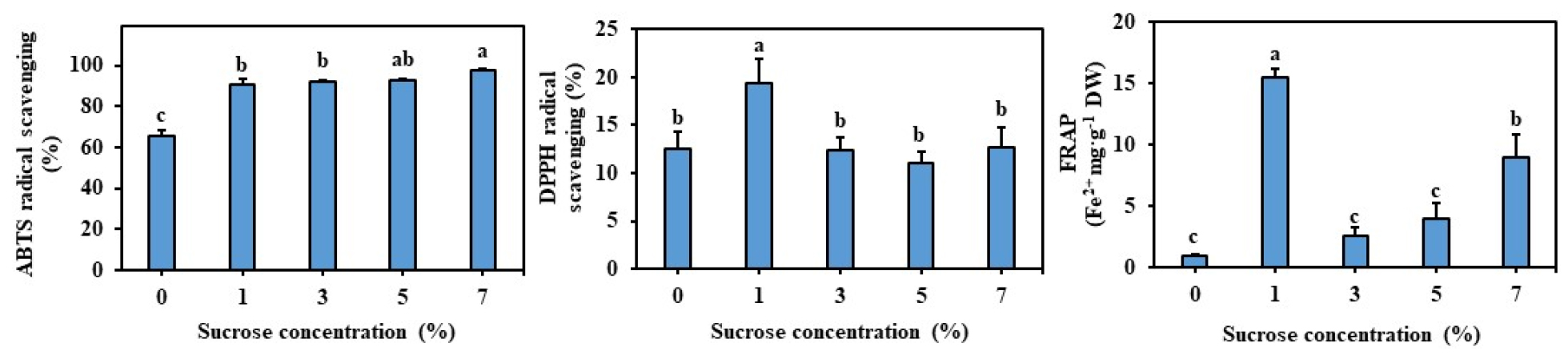
The regenerated plants contained an optimum of 11.52 ㎎ GAE g-1 DW and 1.67 ㎎ CE g-1 DW phenolics and flavonoids respectively on MS medium supplemented with 3% sucrose (Fig. 2). The amount of TFC was significantly higher with the plants regenerated on 1% sucrose (Fig. 2). The antioxidant activities of regenerated plant extract were determined by DPPH, ABTS, and FRAP assays and results are presented in Fig. 3A. The DPPH, and FRAP were higher with the plants regenerated on MS medium with 1% sucrose. Whereas, the average DPPH scavenging activity of plants regenerated on MS medium supplemented with 3% sucrose was 12.44%. Whereas, average ABTS and FRAP activities were 92.15% and 2.57% respectively (Fig. 3B, C).
HPLC analysis of phenolics in the regenerated plants
HPLC analysis of Lobelia chinensis plants exhibited the presence of twelve different phenolic compounds including protocatechuic acid, catechin, phoretic acid, coumaric acid, rutin, ferulic acid, naringin, myricetin, quercetin, luteolin, apigenin, and biochanin (Fig. 4). The Lobelia chinensis plant biomass which was produced in optimized medium viz. MS medium containing 3% sucrose contained 0.45 ㎎ g-1 DW of myricetin and 1.19 ㎎ g-1 DW of catechin (Fig. 5).
Discussion
Micropropagation of medicinal plants through in vitro culture has several advantages as it allows mass generation of plants with genetically identical chemotypes, it allows quality and safety of final products and it proves to be useful in reducing consumption of pressure on potentially rare wild populations (Lee et al., 2022; Moraes et al., 2021). However, micropropagation protocols have to be worked out for specific plant species cultured in vitro to provide mineral nutrients, sources of carbohydrates, and appropriate environmental conditions including light, photoperiod, and temperature to obtain high regeneration rates (Lee et al., 2022; Mathe et al., 2015). Lobelia chinensis is one such plant in which an in vitro regeneration protocol has not been developed for biomass and metabolite production. Therefore, in the current study, we initiated in vitro cultures of Lobelia chinensis using stem segments and worked out various parameters like the effect of various medium compositions, salt strength of the medium, and sucrose concentration on plant regeneration.
Of the various plant tissue culture media tested best growth regeneration of Lobelia chinensis was on the MS medium when compared to B5, SH, WPM, N6, and NLN media, and 4.5 shoots regenerated from nodal explants on the MS medium. The fresh weight and dry weight, shoot length, and leaf area were all optimum with the plant regenerated on MS medium. Similarly, MS medium has been widely used for micropropagation of several medicinal plants such as Andrographis alata (Kadapatti and Murthy, 2021), Artemisia nilagirica (Shinde et al., 2016), Andrographis paniculata (Dandin and Murthy, 2012), Bacopa monnieri (Faisal et al., 2018), Spilanthes oleracea (Dandin et al., 2014), Vitex trifolia (Hiregoudar et al., 2006). However, the Woody plant medium induced the highest response regeneration response in Rauvolfia tetraphyla (Faisal et al., 2012), whereas, the Gamborg (B5) medium had the best morphogenetic response in Saraca asoca (Shirin et al., 2015). We have also tested different strengths of MS medium viz. double, full, half, and quarter-strength for the regeneration of Lobelia chinensis, and the highest response was recorded on full-strength MS medium in terms of fresh weight, and dry weight, shoot number, shoot length, and leaf area, the number of roots and root length. In contrast to the present results, several studies have shown that a half-strength MS medium has resulted in the maximum number of shoots in Mentha spicata (Fedel et al., 2010), and Typhonium flagelliforme (Neto and Otoni, 2003).
Sucrose is used in the plant tissue culture medium as a source of energy and to maintain the osmotic in the medium (Rezali et al., 2017). In the current experiments, 1, 3, 5, and 7% of sucrose was supplemented to MS medium for the regeneration of Lobelia chinensis and results revealed that 3% sucrose was good for the regeneration of plants and accumulation of biomass. Similar to the current observations 3% sucrose was found to be most appropriate for shoot multiplication date palm (Mazri et al., 2016). Different sugars were used as a carbon source in tissue cultures such as fructose, glucose, maltose, and lactose, however, sucrose is the most effective carbon source in the regeneration of medicinal plants in vitro like Indian snakeroot (Alatar, 2015) and wormwood (Bolyard, 2018).
In the current study, we have estimated the content of total phenolics and flavonoids in the regenerated plants and 11.52 ㎎ GAE g-1 DW and 1.67 ㎎ CE g-1 DW phenolics and flavonoids respectively on MS medium supplemented with 3% sucrose. However, total flavonoid accumulation was higher with the medium supplemented with 1% sucrose. Further, analysis of antioxidant activity through DPPH, ABTS, and FRAP methods showed the highest antioxidant activity in the plants grown in the medium supplemented with 1% sucrose. Usually, the higher concentrations of sucrose which lead to elevated osmotic potential and such elevated concetrations were responsible for higher accumulation of phenolics, flavonoids, chlorogenic acid and hypericin in Eleutherococcus sessiliflorus embryogenic cultures (Shohael et al., 2006) and higher concentration of phenolics and flavonoids in Echinacea angustifolia adventitious root cultures (Wu et al., 2006). Whereas, in Hypericum perforatum adventitious root cultures supplementation of 3% sucrose was found optimal for both biomass, phenolic, and flavonoid accumulation (Cui et al., 2010). In the current study, the addition of 3% sucrose was responsible for shoot regeneration and accumulation of plant biomass, however, the accumulation of flavonoids, and antioxidants was optimum with a medium containing 1% sucrose. These results depict that the accumulation of bioactive compounds and antioxidants is dependent on the physiological status of a particular plant species. Li et al. (2015) have demonstrated the potent antioxidant and anti-inflammatory activities of L. chinesis by using in vitro and in vivo models. Therefore, we presume the regenerated plant L. chinesis could be used in pharmaceutical preparations.
The Lobelia chinensis plant biomass produced after plant regeneration was assessed phytochemically using HPLC and the results showed that the plant biomass possessed polyphenolics viz. protocatechuic acid, catechin, phoretic acid, coumaric acid, rutin, ferulic acid, naringin, myricetin, quercetin, luteolin, apigenin, and biochanin. Of all the phenolics the concentration of myricetin (0.45 ㎎ g-1 DW) and catechin (1.19 ㎎ g-1 DW) were in higher concentration when compared to other compounds. Myricetin is considered a dietary molecule possessing a wide range of activities including anti-oxidant, anticancer, antidiabetic, and anti-inflammatory effects (Semwal et al., 2016). Similarly, catechin possesses antibacterial, anti-inflammatory, antidiabetic, and anticancer activities and possesses high therapeutic values (Ganeshpurkar and Saluja, 2020). Therefore, the in vitro regenerated L. chinensis plants could be used for the extraction and utilization of these compounds. Further, L. chinensis plant biomass could be also utilized in the preparation of value-added products.
Successful in vitro plant regeneration of L. chinensis was achieved in the current studies and results reveal that full strength MS medium supplemented with 3% sucrose was found suitable for the regeneration of plants. The regenerated plants possessed higher concentrations of phenolics (11.52 ㎎ GAE g-1 DW) and flavonoids (1.67 ㎎ CE g-1 DW). In addition, DPPH, ABTS, and FRAP assays showed the highest antioxidant activity. HPLC analysis of the L. chinensis plant biomass produced possessed polyphenolics and myricetin and catechin were in higher concentration. Thus in vitro, regenerated L. chinensis could be used as raw material for herbal preparation.