Introduction
Materials and Methods
Animals and Reagents
Preparation of UP
Establishment of UC experimental model and treatment of UP
Disease activity index (DAI)
Cytokine assay
Western blot analysis
Statistical analysis
Results
Effects of UP on weight loss and DAI in DSS-induced colitis in mice
Effects of UP on colon shortening in DSS-induced colitis in mice
Effects of UP on IL-6 and TNF- α levels in DSS-induced colitis in mice
The Effect of UP on Activation of NF-B p65 in DSS-Induced Colitis
Discussion
Introduction
Ulcerative colitis (UC) is a chronic intestine inflammatory disorder and the incidence has risen rapidly worldwide in recent years (Ungaro et al., 2017). The exact pathogenesis of UC is not completely understood, which may derive from genetic susceptibility, microbial infection, dysregulated immune response, and the external environmental factors (Manichanh et al., 2012; Seyedian et al., 2019). The main clinical symptoms of UC are characterized by diarrhea, weight loss, abdominal pain, and intestinal bleeding, resulting in epithelial barrier disruption (Knights et al., 2013).
Increasing evidence suggests that dysregulated inflammatory cytokines including interleukin (IL)‑6 and tumor necrosis factor (TNF)‑α have been implicated in the occurrence and progression of UC (An et al., 2020). An aberrant release of inflammatory cytokines in response to mucosal injury in the lamina propria results in intestinal inflammation (Le Berre et al., 2023). Additionally, excessive oxidative stress during UC induces colonic damage and stimulates the immune cells that release the inflammatory cytokine, resulting in persistent inflammation. Studies currently indicate that mucosa from UC patients exhibits increased levels of IL‑6 and TNF‑α (Vipul et al., 2016). Others reported that inhibiting inflammatory cytokines effectively improved intestinal inflammation (Li et al., 2018). Nuclear factor-kappa B (NF-κB) is a critical nuclear transcription factor implicated in numerous inflammatory diseases, including UC (Zhu et al., 2023). Activation of NF-κB in the intestinal mucosa induces the expression of inflammatory cytokines (Li et al., 2024). Furthermore, clinical studies have verified the activation of NF-κB in colonic tissue from patients with UC (Banskota et al., 2021). Based on these results, there is an interest in searching candidates that are able to inhibit the generation of inflammatory cytokines and activation of NF-κB for the UC treatment. The clinical treatments for UC include glucocorticosteroids, immunomodulators and biological therapies, but these therapies cause severe adverse effects for long-term usage (Salice et al., 2019; Sandborn, 2008). Thus, it is needed to explore alternative treatment for UC. Recently, the importance of marine plants as functional materials in food, health products, and pharmaceuticals is well known due to its beneficial activities. Undaria pinnatifida (UP), a brown alga, exhibits beneficial biological property including anti-oxidative, anti-tumor, and anti-angiogenic effect (Etman et al., 2021; Nadeeshani et al., 2022). Recent studies have reported that UP inhibited allergic inflammation by modulating MAPK/ NF-κB pathways (Yu et al., 2024). Additionally, UP suppressed the H2O2-induced oxidative stress in Vero cells (Lee et al., 2023). However, the protective mechanism of UP on UC remain unknown. Since DSS is directly toxic to gut epithelial cells of crypts and affects the mucosal barrier, clinical symptoms are similar to those of human colitis (Boirivant and Cossu, 2012; Wirtz et al., 2007). Hence, the DSS-induced colitis model is particularly useful for exploring the inflammatory mechanisms in colitis. To provide experimental evidence that UP might be a useful treatment for UC, we investigated the anti-colitis effects and mechanism of UP on DSS-induced colitis in mice.
Materials and Methods
Animals and Reagents
BALB/c mice (male), aged 7 weeks old, were procured from Hyochang Science (Daegu, Korea). The mice were maintained in a pathogen-free environment and kept under a 12-hour light–dark cycle at room humidity (50 ± 10%) and temperature (23 ± 2°C). DSS was purchased from MP Biomedical (Santa Ana, CA, USA). The bicinchoninic acid (BCA) and enhanced chemiluminescence (ECL) reagent were obtained from Thermo Scientific Inc. (Rockford, IL, USA). Anti-mouse IL-6/ TNF-a assay kits were purchased from BD Pharmingen (San Diego, CA, USA). NF-κB antibodies (Abs) were procured from Santa Cruz Biotechnology (Dallas, TX,, USA). Animal experiment was conducted in accordance with the internationally accepted principles in Daegu Haany university guidelines (DHU2020-075).
Preparation of UP
Dried UP was obtained from a local market (Gijang, Korea). Dried UP was pulverized into a fine powder decocted in distilled water (1 L) for 3 h, and concentrated under vacuum rotary evaporator. The extract was filtered, freeze dried (Eyela, FDU-2000, Japan) and stored at –20°C (yield, 6.84%). The extract was dissolved in distilled water and filtered through a 0.22 ㎜ syringe filter.
Establishment of UC experimental model and treatment of UP
Colitis in mice was induced by treatment daily with DSS 5% (w/v) drinking water for 7 days. Mice were randomized into 4 groups (n=6/ group): Group I-control group (without DSS colitis), Group II-DSS alone group, Group III-DSS+ UP (10 ㎎/㎏), Group IV-DSS + UP (100 ㎎/㎏). UP extract was orally administered once a day during DSS treatment and after which the mice were sacrificed. The mice were monitored daily for weight loss, severity of bleeding and stool consistency. Mice were sacrificed after treatment and the colon and blood was corrected for inflammatory mediators’ analysis.
Disease activity index (DAI)
Intestinal disease activity was assessed based on weight loss, the presence of diarrhea accompanied by blood and mucus, and colonic shortening (Eichele et al., 2017). the severity of colitis was assessed daily using the DAI, scoring which includes three parts as reported by Munyaka et al. (2016): body weight loss (0–4), stool consistency (0–4) and degree of intestinal bleeding (0–4). The DAI values were measured by investigators blinded.
Cytokine assay
The levels of IL-6 and TNF-α in the colon tissues and serum of indicated groups were measured by using a modification of an ELISA (Yang and Myung, 2023). Briefly, plates (96-well) were coated with IL-6 and TNF-a primary (monoclonal) Abs and then reacted overnight at 4°C. After washes, standard solution of IL-6 and TNF-a or sample were incubated. After washing with PBST, the plate was incubated to biotinylated (secondary) IL-6 and TNF-a Abs followed by incubation for 1 h. After further washing, AP and substrates were sequentially mixed and the reaction stopped. The amount of IL-6, and TNF-α at 405 ㎚ were analyzed using an ELISA reader.
Western blot analysis
Nuclear lysates of distal colons were prepared using nuclear extraction reagent according to the manufacturer’s instructions. After protein quantification using BCA reagent, samples were added with sample buffer, separated by gel electrophoresis, and transferred onto membranes. Membrane was blocked by 5% skimmed milk for 1h and subsequently reacted with NF-κB (p65) primary Abs at 4°C for 24 h. After washing, membranes were reacted with secondary Abs for 2 h. After further washing, proteins were visualized using an ECL detection kit.
Statistical analysis
Results are shown as the mean ± SD of three experiments. Statistical analyses were conducted using one-way ANOVA with a Tukey post hoc test. The significance levels between experimental groups were set at P < 0.05.
Results
Effects of UP on weight loss and DAI in DSS-induced colitis in mice
DSS-induced colitis experimental model has a phenotype similar to that of human UC (Wirtz et al., 2007). To induce acute colitis, mice were treated by 5% DSS for 7 days and clinical symptoms of colitis monitored daily. First, we investigated whether UP at the doses 10 and 100 ㎎/㎏ can alleviate the DSS-induced weight loss in colitis. The results illustrated that the body weight of mice treated with DSS significantly reduced compared to the control. However, the UP (10 and 100 ㎎/㎏) treatment improved the DSS-induced the weight loss in mice (Fig. 1A).
The common feature of colitis is an increase in DAI (Eichele et al., 2017). Generally, DAI was calculated based on body weight loss, diarrhea and rectal bleeding degree. We investigated the regulatory effect of UP at the doses (10 and 100 ㎎/㎏) on DSS-induced the DAI score increase in mice. We observed that the DAI scores with DSS significantly attenuated compared to the control. However, UP (10 and 100 ㎎/㎏) markedly decreased the DSS-enhanced DAI combined scores (Fig. 1B). These results indicate that UP can efficiently relieve the DSS- induced clinical symptom in mice.
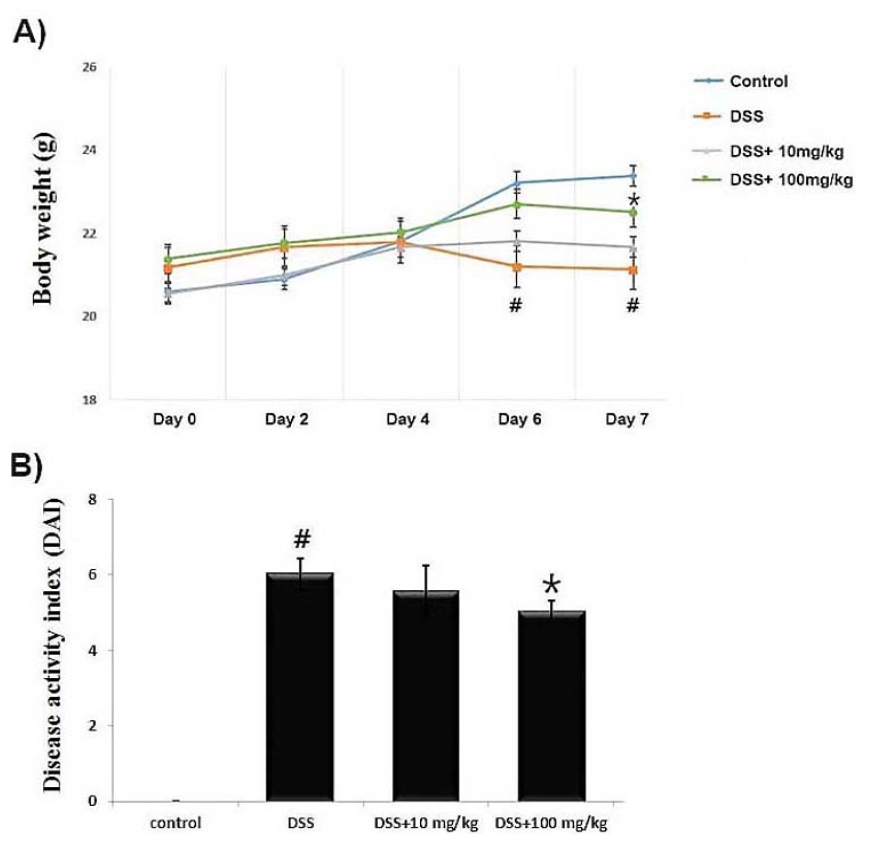
Fig. 1.
Effect of UP on DSS-induced the body weight loss in mice. Experimental colitis was induced by administering a 5% DSS in the drinking water for 7 days. UP (10 and 100 ㎎/㎏) were orally administered for over the same period. (A) Body weight of mice was measured at the same time on the experimental days. (B) DAI was scored as described in Materials and Methods. Data were represented in the mean ± SD of experiments (n = 6) (#P < 0.05 versus control and *P< 0.05 versus DSS alone).
Effects of UP on colon shortening in DSS-induced colitis in mice
The degree of colonic length change has been used as a parameter for the severity of intestinal inflammation (Hendrickson et al., 2002). We evaluated the beneficial effect of UP on the DSS-induced colonic length change in mice. As shown in Fig. 2A, at days 7 following DSS treatment, we observed that colon lengths in the DSS-administered mice were significantly shorter (5.02 ± 0.41 ㎝) than those of control (8.45 ± 0.32 ㎝). However, UP at the doses 100 ㎎/㎏ attenuated the DSS-induced the colon shortening (6.52 ± 0.47 ㎝). Relative colon length is shown in Fig. 2B.
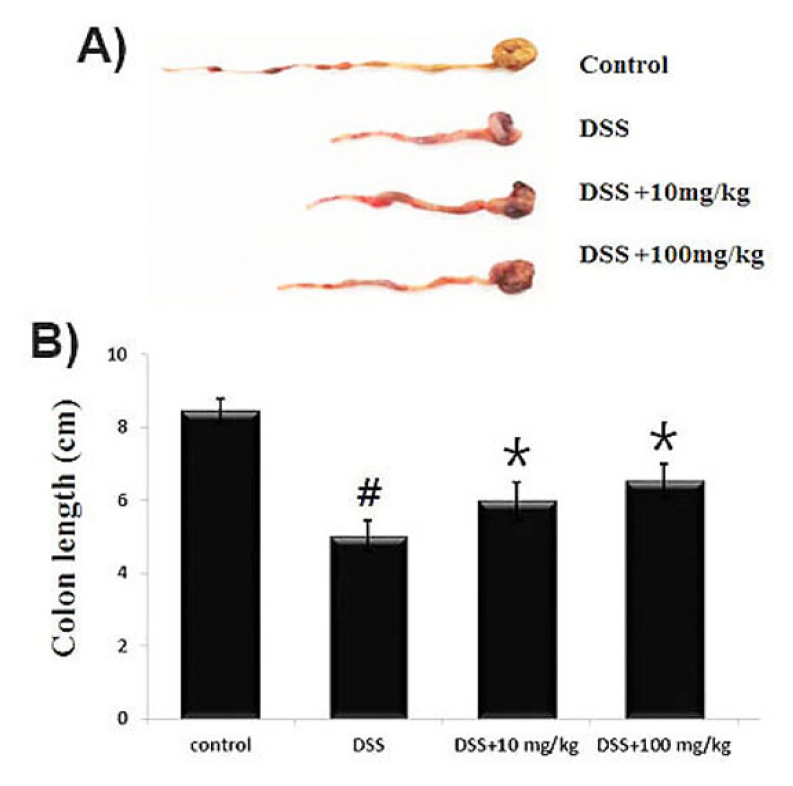
Fig. 2.
Effect of UP on DSS-induced the colon length shortening and DAI in mice. Experimental colitis was induced by administering a 5% DSS in the drinking water for 7 days. UP (10 and 100 ㎎/㎏) were orally administered for over the same period. (A) The colons were removed at day 7 after DSS treatment, and the colon lengths were measured. (B) Relative colon lengths were represented. Data were shown in the mean ± SD of experiments (#P < 0.05 versus control and *P< 0.05 versus DSS alone).
Effects of UP on IL-6 and TNF- α levels in DSS-induced colitis in mice
Dysregulated inflammatory cytokine is implicated in physiological processes and pathogenesis of UC (Ungaro et al., 2017). In this study, we evaluated the effect of UP on DSS- induced inflammatory cytokine secretion in colon tissue and serum using ELISA. The results in Fig. 3A showed that the IL-6 and TNF-α levels significantly increased by DSS, but the supplementation of UP at the doses 10 and 100 ㎎/㎏ markedly decreased IL-6 and TNF- α levels in colons. The rate of inhibition of IL-6 and TNF- α levels by UP at the doses 100 ㎎/㎏ was 20.7% and 23.9%, respectively.
We also assessed the effects of UP on serum levels of IL-6 and TNF-α in DSS-induced colitis. Blood samples were collected and serum IL-6 and TNF-α levels were measured using ELISA. The results show that UP at the doses 10 and 100 ㎎/㎏ alleviated the DSS-enhanced IL-6 and TNF- α serum levels in mice. The rate of inhibition of IL-6 and TNF-a levels by UP at the doses 100 ㎎/㎏ was 23.8% and 26.6%, respectively.
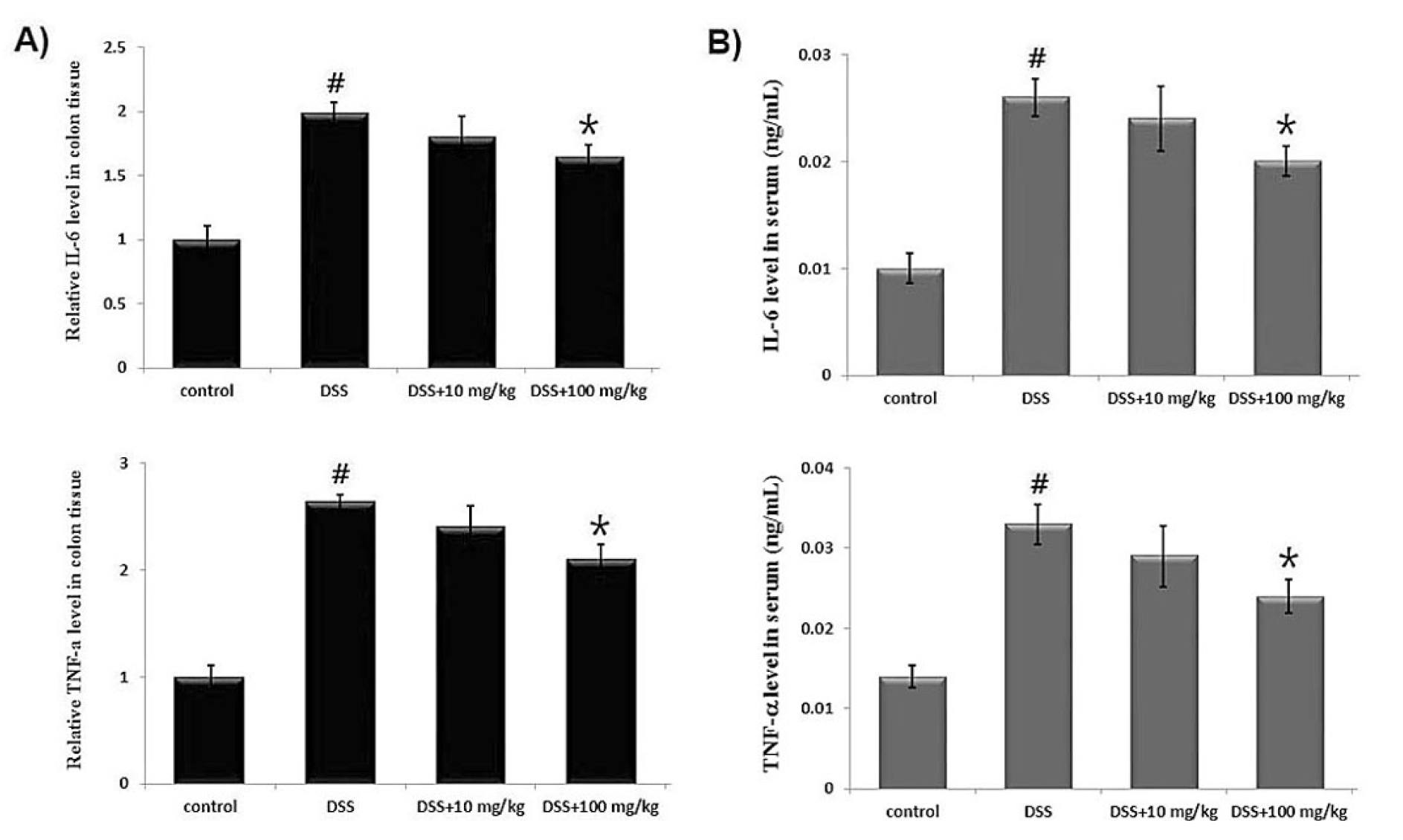
Fig. 3.
Effect of UP on the IL-6 and TNF- α levels in DSS-induced colitis tissue and serum. Experimental colitis was induced by administering a 5% DSS in the drinking water for 7days. UP (10 and 100 ㎎/㎏) were orally administered for over the same period. At the end of the experiment, the colon tissues were excised and homogenized. (A) The amount of IL-6 and TNF- α in colonic tissue (A) and serum (B)were assayed by ELISA kits. Results were represented in the mean ± SD of experiments (#P < 0.05 versus control and *P< 0.05 versus DSS alone).
The Effect of UP on Activation of NF-B p65 in DSS-Induced Colitis
NF-B activation is implicated in the severity of intestinal inflammation through the regulation of inflammatory cytokines. To investigate the anti-colitis mechanism of UP, we assessed whether UP attenuated the activation of NF-B p65 in colon tissues. The result showed that activation of NF-B p65 was significantly increased in the colon tissues of DSS-treated mice compared to the control. In contrast, UP at the doses 10 and 100 ㎎/㎏ exhibited a significant inhibition on the activation of NF-B p65 in the colons of colitis mice. The relative level of NF-B p65 was measured using an image analyzer (Fig. 4). The rate of inhibition of NF-B activation by UP at the doses 100 ㎎/㎏ was 26.3 %.
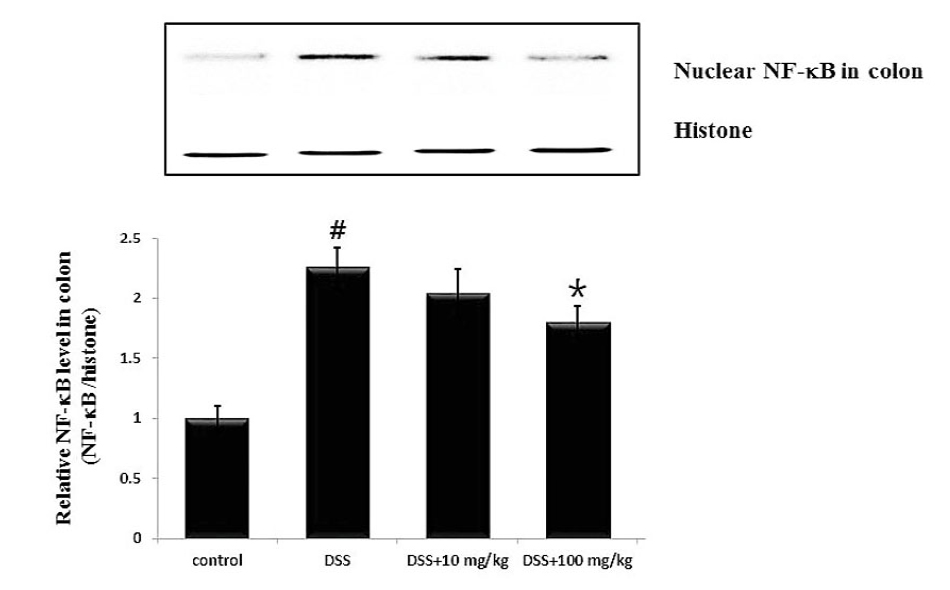
Fig. 4.
Effect of UP on the NF-kB activation in in DSS-treated colon tissue. At the End of the experiment, the colon tissues were excised and homogenized. Nuclear extracts in colonic tissue were prepared by nuclear extraction reagent kit. (A) The levels of NF-kB p65 were assayed by Western blot analysis. (B) The relative ratio of NF-kB was calculated using an image analyzer. Data were represented in the mean ± SD of experiments (#P < 0.05 versus control and *P< 0.05 versus DSS alone).
Discussion
Although corticosteroid is effective in clinical remission, severe adverse effect can lead to their discontinuation; hence, alternative treatments are needed. In this study, we attained to provide experimental evidence that UP might be a useful therapeutic agent for UC. The finding of this study demonstrated that UP alleviate DSS-induced the colitis clinical signs and inflammatory response, suggested a molecular mechanism by which UP inhibits intestinal inflammation.
UC is characterized by chronic inflammation of the intestinal tract, and mucosal immunity, intestinal environment, and external stimulation; many other complicated factors are also related with the pathogenesis of UC (Seyedian et al., 2019). The current therapeutic drugs of UC include corticosteroid, immunosuppressant, and so on, but, these cause serious side effects for long-term usage (Isaacs et al.,, 2005; Salice et al., 2019). Thus, it is needed to explore alternative treatment for UC. Recently, marine plants have gained attention in developing the treatment of UC. Hence, this study evaluated the improving effect and mechanism of UP in DSS-induced colitis. The important clinical signs of UC were body weight loss, diarrhea and bloody stools (Eichele et al., 2017; Munyaka et al., 2016). In this study, the results showed that UP treatment (10 and 100 ㎎/㎏) markedly alleviated DSS-enhanced weight loss and colon shortening in mice. Generally, the severity of colitis was assessed based on DAI scores of body weight loss, diarrhea and bleeding degree. We observed that DSS-induced the DAI values were significantly alleviated in groups administered UP. These results indicated that UP exerts an anti-colitis activity through regulation the clinical symptoms of colitis caused by DSS.
Accumulated experimental evidence shows that dysregulated inflammatory cytokines are implicated in the pathogenesis of intestinal inflammation (Xiong et al., 2021). It was reported that IL-6 and TNF- α levels are elevated in patients with UC and plays a key role in UC pathogenesis (Ungaro et al., 2017). Thus, we tried to determine the ameliorative effect of UP on DSS-induced the IL-6 and TNF- α levels in colitis tissue and serum. The results show that UP treatment inhibited the DSS- enhanced IL-6 and TNF- α levels in colitis tissue and serum. From this, we suggest that the anti-colitis activity of UP may be associated with the attenuation of inflammatory cytokines in DSS-induced colitis mice.
NF-κB is related to the inflammatory response in UC (Zhu et al., 2023). NF-B activation is implicated in the severity of intestinal inflammation through the regulation of inflammatory cytokines. It was also reported that NF-κB activation induced the disruption of epithelial barrier function. Therefore, suppression of NF-κB activation has been demonstrated as an anti-inflammatory strategy in treatment of UC (Banskota et al., 2021). In this study the results in Fig. 4 show that UP markedly decreased the DSS-enhanced NF-κB p65 activation in colons. These results indicate that UP attenuated the symptoms of UC and intestinal inflammation through blocking NF-κB activation in DSS-induced colitis. Although we reveal the role of UP treatment in the attenuation of NF-κB activation in colitis, the regulatory effects of UP on degradation and phosphorylation of Iκb-α was not elucidated. Therefore, further study must be performed to elucidate the precise mechanism of UP in the attenuation of intestinal inflammatory response.
UP contains various bioactive ingredients, such as fucoidan, polysaccharides, polyphenols, vitamins and protein (Murphy et al., 2017; Wang et al., 2018). It was reported that fucoidan has anti-oxidant property by reducing H2O2-induced oxidative stress in osteoblast (de Melo et al., 2022). Fucoidan alleviates lung inflammation through regulating Nrf2 signaling pathway (Zhu et al., 2020). Damage to intestinal epithelial and destruction of the gut mucosal barrier, leading to release of various inflammatory cytokines. Additionally, excessive oxidative stress during UC induces colonic damage and stimulates the secretion the inflammatory cytokine, resulting in persistent inflammation. Hence, agents with anti-inflammatory and anti-oxidant properties can be used to develop colitis therapy (Xiong et al., 2021). As UP and its ingredients have antioxidant and anti-inflammatory effects, we suggested that UP could represent a novel nutraceutical option for the management of UC.
In conclusion, our results show that UP possess an anti-colitis activity by regulation of the clinical symptoms against DSS-induced colitis in mice. In addition, we demonstrate that the anti-inflammatory effect and mechanism of UP can attributed to the suppression of inflammatory cytokines (IL-6 and TNF- α) and NF-κB activation in colon tissue. These finding provide experimental evidence that UP might be a promising therapeutic candidate for treating UC.




