Introduction
Materials and Methods
Chemical reagents and Antibodies
Sample Extraction
3T3-L1 Cell Culture
Differentiation of 3T3-L1 Cells
Measurement of Cell Number and Viability
Oil-Red O staining
Measurement of Glycerol Content
Western Blot Analysis
Statistical Analysis
Results
Effect of RAL on lipid droplet accumulation in 3T3-L1 cells
Effect of RAL on lipolysis in 3T3-L1 cells
Effect of RAL on thermogenesis in 3T3-L1 cells
Effect of AMPK activation on RAL-mediated increases in protein level associated with lipolysis and thermogenesis in 3T3-L1 cells
Discussion
Introduction
Obesity, characterized by excessive accumulation of body fat, has been recognized as a significant health issue due to its association with various disorders, including musculoskeletal disorders, cardiovascular diseases, diabetes, and cancer (Geum et al., 2023; Wlodarczyk and Slizewska, 2021). Furthermore, the recent COVID-19 pandemic, which has become a serious global issue, has led to a rapid increase in the obese population (Abbas et al., 2020). It has been reported that obese individuals have a higher risk of infection with COVID-19 (Michalakis and Ilias, 2020). Due to the various health problems caused by obesity, pharmacotherapy is considered a major strategy for treating obesity (Fu et al., 2016). However, the chemical drugs currently used for obesity treatment have serious side effects, highlighting the urgent need for the development of natural remedies without side effects (Fu et al., 2016).
Natural plant resources serve as a foundation for the development of materials for combating obesity (Karris et al., 2019). Therefore, functional foods and dietary supplements using natural plant resources are being developed for the prevention and treatment of obesity (Sander et al., 2020). The Rosa acicularis Lindl., also known as the prickly rose, is a deciduous shrub that belongs to the Rosaceae family. It is found naturally in various regions, including China, Japan, Russia, Mongolia, Kazakhstan, northern Europe, North America, and Korea. The leaves, flowers, roots, and fruits of Rosa acicularis have been used as food and medicinal plants (Olennikov et al., 2021). Historically, the fruit of Rosa acicularis has been utilized for its potential benefits in gum strengthening and treating heart ailments, whereas the branches have been employed to prevent diarrhea and intestinal disorders. Additionally, the bark of Rosa acicularis stem has been used as a detoxifying agent and for addressing lymphatic system disorders, while the leaves have been traditionally used as a diuretic (Olennikov et al., 2021). According to recent research, Rosa acicularis has been found to possess antioxidant properties, inhibit lipase activity, and exhibit antimicrobial activity (Olennikov et al., 2021).
Although Rosa acicularis has been shown to inhibit lipase activity, indicating its potential as an anti-obesity agent, the precise mechanism of action has yet to be fully understood due to a lack of research. Therefore, in this study, we aimed to investigate the anti-obesity properties of Rosa acicularis leaves and elucidate their mechanism of action by using 3T3-L1 adipocytes.
Materials and Methods
Chemical reagents and Antibodies
The differentiation inducers (dexamethasone, IBMX, and insulin) for the maturation of preadipocyte cells into mature adipocytes, Oil Red O for lipid droplet staining and Compound C for the inhibition of AMPK were purchased from Sigma-Aldrich (St. Louis, MO, USA). The primary antibodies against adipose triacylglycerol lipase (ATGL, #2138), hormone-sensitive lipase (HSL, #4107), p-HSL (#4137), perilipin-1 (#9349), AMP-activated protein kinase (AMPK, #5831), p-AMPK (#2535), uncoupling protein 1 (UCP-1, #14670), and β-actin (#5125), and secondary antibodies such as anti-rabbit IgG, HRP-linked Antibody (#7074) and anti-mouse IgG, HRP-linked Antibody (#7076) were purchased from Cell Signaling (Beverly, MA, USA). The primary antibody against peroxisome proliferator-activated receptor-gamma coactivator-1α (PGC-1α, sc-518025) was purchased from Santa Cruz Biotechnology (Dallas, TX, USA).
Sample Extraction
Rosa acicularis leaves used in this study were provided by Forest Medicinal Resources Research Center (Yeongju, Korea) after taxonomic identification. To prepare the extract of Rosa acicularis leaves, 10 g of powdered Rosa acicularis leaves were soaked in a 20-fold volume of distilled water at 40℃ for 24 h for the extraction. After centrifugation at 15,000 rpm, the obtained supernatant was freeze-dried to obtain the final analytical sample, which is Rosa acicularis leave extracts (RAL). RAL was stored at -80℃ until analysis and dissolved in distilled water for cell treatment.
3T3-L1 Cell Culture
To culture the preadipocyte 3T3-L1 cells, cells were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA) and then incubated in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% bovine calf serum (BCS) and 1% penicillin-streptomycin at 37°C under a 5% CO2 atmosphere.
Differentiation of 3T3-L1 Cells
At 2 days after the preadipocyte 3T3-L1 cells reached 100% confluence in the wells (D0), the 3T3-L1 cells were cultured in DMEM/10% fetal bovine serum (FBS) medium containing DMI (1 μM dexamethasone, 0.5 mM IBMX, and 10 ㎍/mL insulin) for 2 days (D2). Subsequently, the 3T3-L1 cells were incubated in DMEM/10% FBS medium containing insulin (10 ㎍/mL) for an additional 2 days (D4). Afterward, the 3T3-L1 cells were cultured in DMEM/10% FBS medium with media changes every 2 days (D6 and D8).
Measurement of Cell Number and Viability
During the differentiation or the undifferentiation process of 3T3-L1 cells, the cells were treated with RAL for D0-D8. Then, the cell number and viability were measured using NucleoCounter NC-250 (Chemometec, Allerod, Denmark) according to the manufacturer’s protocol.
Oil-Red O staining
After 8 days (D8) of differentiation induction, the 3T3-L1 cells were washed thrice with 1 X PBS and then treated with 10% formalin at room temperature for 1 h for cell fixation. After cell fixation, the 3T3-L1 cells were washed with distilled water and then treated with 60% isopropanol at room temperature for 5 min for dehydration. After that, the 3T3-L1 cells were stained with an Oil-Red O staining solution at room temperature for 20 min to visualize lipid droplets. After lipid staining, 3T3-L1 cells were washed with distilled water five times to remove the unbound Oil-Red O solution, and the stained lipid droplets within 3T3-L1 cells were observed using a light microscope (Olympus, Tokyo, Japan). After observing lipid droplets, the 3T3-L1 cells were air-dried and treated with 100% isopropanol to extract Oil-Red O stained in the lipid droplets, and the absorbance was measured at 500 ㎚ using a microplate reader (Human Cop., Xma-3000PC, Seoul, Korea).
Measurement of Glycerol Content
The free glycerol content was measured using a cell-based glycerol assay kit (Cayman Chemical, Ann Arbor, MI, USA) according to the protocol provided by the manufacturer. After treating the differentiated 3T3-L1 cells with RAL for 2 days, the cell culture media was mixed with reconstituted free glycerol assay reagent at a 1:4 ratio and incubated at room temperature for 15 min. After the reaction, the absorbance was measured at 540 ㎚ using a microplate reader (Human Cop., Xma-3000PC, Seoul, Korea).
Western Blot Analysis
The cells were collected using RIPA buffer and left to stand at 4℃ for 30 min. After centrifugation at 1,5000 rpm for 30 min, the protein extract was obtained. After protein quantification analysis using the BCA protein assay kit (Thermo Fisher Scientific, Waltham, MA USA), an equal amount of protein (30 ㎍/well) was subjected to electrophoresis on a 12% or 8% acrylamide gel at 150 V and 400 A for 1 h. Afterward, the separated proteins on the acrylamide gel were transferred onto a nitrocellulose membrane (Thermo Fisher Scientific) for 2 h at 100 A and 300 A. After blocking at room temperature for 1 h, the membranes were incubated with the primary antibodies (1:1,000) at 4℃ overnight. After that, the membranes were incubated with the secondary antibodies (1:1000) at room temperature for 1 h. After treating ECL Western blotting substrate on the membrane, the protein band was visualized using LI-COR C-DiGit Blot Scanner (LI-COR, NE, USA). Quantitative analysis of visualized protein bands was performed using the software UN-SCAN-IT gel version 5.1 (Silk Scientific Inc. Orem, UT, USA).
Statistical Analysis
All experiments were repeated at least thrice. Statistical analyses were verified using GraphPad Prism version 5.0 (GraphPad Software, Inc.) and data are presented as mean ± standard deviation. Results with *P<0.05 were considered statistically significant. Each data point was analyzed using a one-way analysis of variance and the data was analyzed using the Bonferroni post hoc test.
Results
Effect of RAL on lipid droplet accumulation in 3T3-L1 cells
We investigated the inhibitory activity of RAL on lipid droplet accumulation in differentiated 3T3-L1 cells to determine the anti-obesity activity of RAL. As shown in Fig. 1A, RAL was treated to 3T3-L1 cells (D0-D8) during the differentiation of 3T3-L1 cells with DMI and insulin. As a result (Fig. 1B), compared to the non-treated group (CON), quite a lot of lipid droplets were accumulated in 3T3-L1 cells differentiated with DMI and insulin without RAL treatment. However, treatment with RAL during differentiation of 3T3-L1 cells with DMI and insulin markedly reduced lipid droplet accumulation. In addition, RAL dose-dependently reduced triacylglycerol (Fig. 1C). We treated RAL with or without the presence of DMI and insulin to verify its cytotoxicity towards 3T3-L1 cells and measured the cell number and cell viability. As shown in Fig. 1D, there was no effect of RAL on the number and viability of 3T3-L1 cells when DMI and insulin were absent. However, treatment of RAL during the differentiation process of 3T3-L1 cells induced by DMI and insulin had no effect on cell viability but reduced the increase in cell number.

Fig. 1.
Effect of RAL on the accumulation of lipid droplets and triacylglycerol in 3T3-L1 cells. (A) Experimental design. (B) Oil-Red O staining in 3T3-L1 cells treated with RAL (D0-D8) under the differentiation. CON, control group representing undifferentiated 3T3-L1 cells. (C) Analysis of triacylglycerol (TG) content in 3T3-L1 cells treated with RAL (D0-D8) under the differentiation. CON, control group without RAL treatment in differentiated 3T3-L1 cells. (D) Analysis of cell number and cell viability in 3T3-L1 cells treated with RAL (D0-D8) under the undifferentiation and the differentiation). *P < 0.05 vs. CON group. DMI, mixture of 0.05 mM IBMX, 1 μM dexamethasone and 10 ㎍/mL insulin; RAL, Rosa acicularis leaves extracts; TG, triacylglycerol.
Effect of RAL on lipolysis in 3T3-L1 cells
To verify if RAL induces lipolysis, 3T3-L1 cells were treated with RAL (D0-D8) during the differentiation process, and the regulation of lipolysis-related molecules was analyzed by Western blot analysis (Fig. 2A). As shown in Fig. 2A, RAL upregulated the level of adipose triglyceride lipase (ATGL), hormone-sensitive lipase (HSL), and HSL phosphorylation and decreased perilipin-1 level. To further verify if RAL also degrades the accumulated lipids in mature adipocytes, mature 3T3-L1 cells differentiated from preadipocytes were treated with RAL (D8-D10) (Fig. 2B). As shown in Fig. 2B, As shown in Fig. 2B, RAL treatment resulted in a decrease in lipid droplet accumulation and an increase in free glycerol content in 3T3-L1 cells. Furthermore, RAL increased the level of ATGL and HSL protein.
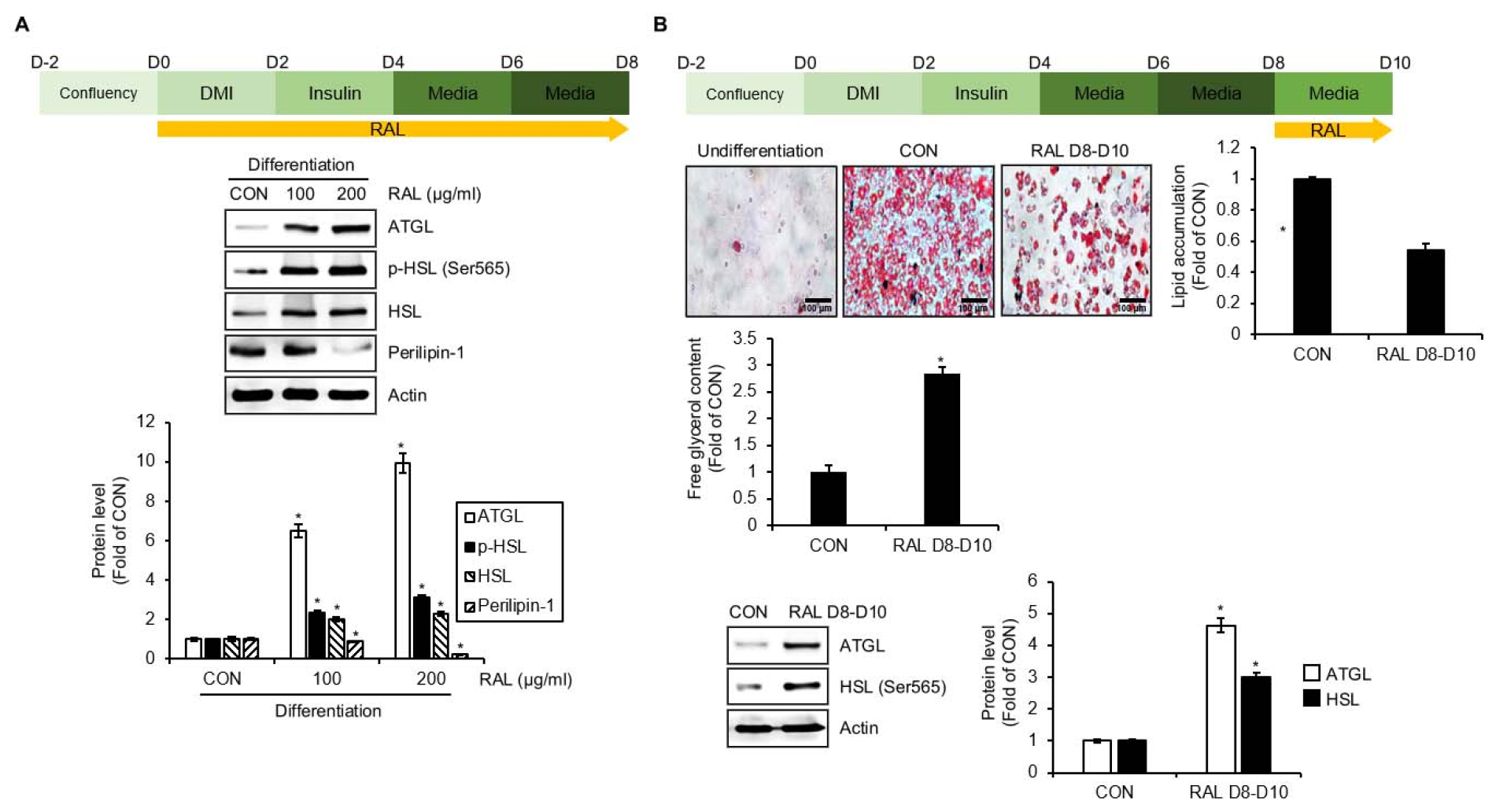
Fig. 2.
Effect of RAL on lipolysis in 3T3-L1 cells. (A) Experimental design and Western blot analysis in 3T3-L1 cells treated with RAL (D0-D8) under the differentiation. (B) Experimental design and Oil-Red O staining, analysis of free glycerol content, and Western blot analysis in the differentiated 3T3-L1 cells treated with RAL (D8-D10). *P < 0.05 vs. CON group. DMI, mixture of 0.05 mM IBMX, 1 μM dexamethasone and 10 ㎍/mL insulin; RAL, Rosa acicularis leaves extracts; ATGL, adipose triglyceride lipase; p-, phosphorylated; HSL, hormone-sensitive lipase.
Effect of RAL on thermogenesis in 3T3-L1 cells
To verify if RAL induces thermogenesis, 3T3-L1 cells were treated with RAL (D0-D8) during the differentiation process, and the regulation of thermogenesis-related molecules was analyzed by Western blot analysis (Fig. 3A). As shown in Fig. 3B, RAL increased the phosphorylation of AMP-activated protein kinase (AMPK), peroxisome proliferator-activated receptor gamma (PPARγ), uncoupling protein 1 (UCP-1), and peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α).

Fig. 3.
Effect of RAL on thermogenesis in 3T3-L1 cells. (A) Experimental design. (B) Western blot analysis in 3T3-L1 cells treated with RAL (D0-D8) under the differentiation. *P < 0.05 vs. CON group. DMI, mixture of 0.05 mM IBMX, 1 μM dexamethasone and 10 ㎍/mL insulin; RAL, Rosa acicularis leaves extracts; p-, phosphorylated; AMPK, AMP-activated protein kinase; PPARγ, peroxisome proliferator-activated receptor gamma; UCP-1, uncoupling protein 1; PGC-1α, peroxisome proliferator-activated receptor-γ coactivator 1α.
Effect of AMPK activation on RAL-mediated increases in protein level associated with lipolysis and thermogenesis in 3T3-L1 cells
To investigate whether AMPK activation is involved in the increase of proteins associated with lipolysis and adipocyte browning mediated by RAL, 3T3-L1 cells were treated with Compound C (CC) to inhibit AMPK activation, followed by treatment with RAL. As shown in Fig. 4A, RAL significantly increased the protein levels associated with lipolysis, including ATGL and HSL, as well as those associated with adipocyte browning, including PPAR and UCP-1, in the absence of CC. However, the presence of CC reduced the increase of these protein levels induced by RAL.

Fig. 4.
Effect of AMPK activation in RAL-induced lipolysis and thermogenesis in 3T3-L1 cells. (A) Western blot analysis in 3T3-L1 cells treated with RAL (D0-D4) in the presence of Compound C (AMPK inhibitor) (D0-D4). (B) The potential mechanism of RAL for anti-obesity. *P < 0.05 vs. the group without RAL treatment. RAL, Rosa acicularis leaves extracts; ATGL, adipose triglyceride lipase; HSL, hormone-sensitive lipase; PPARγ, peroxisome proliferator-activated receptor gamma; UCP-1, uncoupling protein 1.
Discussion
Obesity or the overweight population is on a swift rise across the globe (Park et al., 2020). Obesity is recognized as a social health problem due to its association with various metabolic disorders, and as a result, there is ongoing development of anti-obesity drugs. Orlistat and sibutramine are the main anti-obesity drugs that have been approved by the Food and Drug Administration (Hofbauer et al., 2007). However, long-term use of orlistat, which inhibits digestive lipase activity, and sibutramine, which suppresses appetite, is associated with several side effects, including indigestion, diarrhea, and insomnia (Cheung et al., 2013). Therefore, continuous efforts are underway to develop anti-obesity drugs using natural products to minimize side effects (Park et al., 2020). Functional food agents utilizing non-toxic plant extracts are gaining interest as an attractive alternative drug for reducing obesity (Park et al., 2020). It has been reported that Rosa acicularis has anti-obesity activity by inhibiting the digestive lipase activity (Olennikov et al., 2021). However, there is a lack of clear in vitro studies regarding the anti-obesity activity of Rosa acicularis. Therefore, in this study, we aimed to verify the anti-obesity activity of Rosa acicularis leaves (RAL) and elucidate its mechanism of action in 3T3-L1 preadipocytes.
In this study, we observed that RAL effectively reduces lipid droplet formation and triacylglycerol accumulation in 3T3-L1 cells differentiated with DMI and insulin. In addition, while RAL did not affect the proliferation and viability of undifferentiated 3T3-L1 cells, it reduced the proliferation of differentiated 3T3-L1 cells. These results demonstrate the anti-obesity activity of RAL.
Obesity refers to a state of excessive lipid accumulation, which is known to result from an imbalance between lipid synthesis and lipid breakdown (Lipolysis) (Kersten, 2001). Lipolysis, the process by which triacylglycerol is broken down into free fatty acids, is considered a key process for weight loss (Duncan et al., 2007). Among various enzymes involved in lipid metabolism, ATGL and HSL are important regulators of the lipolysis process that hydrolyze intracellular triacylglycerol into glycerol and free fatty acids (Kratky et al., 2014). ATGL cleaves one fatty acid from triglycerides to convert them into diacylglycerol, while HSL cleaves the fatty acids of diacylglycerol to convert them into glycerol and free fatty acids (Gaidhu et al., 2010). In addition, perilipin-1, which encircles the lipid droplets within adipocytes, is also known to have a significant impact on the process of lipolysis (Duncan et al., 2007). The decrease of perilipin-1 has been demonstrated to promote lipolysis through various in vitro and in vivo studies (Martinez-Botas et al., 2000; Souza et al., 2002; Tansey et al., 2003). These previous reports suggest that the increase of ATGL and HSL, and the decrease of perilipin-1 can promote the process of lipolysis. The present study revealed that RAL treatment during the differentiation process of 3T3-L1 cells resulted in an increase in ATGL, p-HSL, and HSL, and a decrease in perilipin-1. The changes in these proteins induced by RAL treatment indirectly indicate that RAL can induce lipolysis. To confirm if RAL induces lipolysis, fully differentiated 3T3-L1 cells were treated with RAL. As a result, we observed a decrease in lipid droplets, an increase in glycerol content, and an increase in ATGL and HSL proteins in 3T3-L1 cells treated with RAL. These findings provide evidence that RAL can effectively induce lipolysis in vitro. Obesity can also result from excessive energy intake without energy expenditure (Levin and Sullivan, 1984). Thermogenesis is a process in which accumulated energy is consumed as heat, and increasing thermogenesis is considered an effective tool for treating obesity (Pan et al., 2020). There is evidence that thermogenesis is reduced in overweight or obese individuals (Levin and Sullivan, 1984). It has been reported that the activation of AMPK, which is involved in energy homeostasis, promotes energy expenditure resulting in the inhibition of weight gain in high-fat diet animal models (Pollard et al., 2019). PPARγ plays an important regulatory role in the differentiation of not only white adipocytes but also brown adipocytes, and recent studies have reported that it induces thermogenesis in white and brown adipose tissue in obese animal models (Sell et al., 2004). And UCP-1 and PGC-1α play a role in dissipating stored energy in white adipose tissue as heat through thermogenesis (Cannon and Nedergaard, 2004; Lo and Sun, 2013). Thus, AMPK, PPARγ, UCP-1, and PGC-1α have been identified as important targets for developing anti-obesity drugs that target thermogenesis (Pan et al., 2020). In this study, we observed that the treatment of RAL in differentiating 3T3-L1 cells resulted in an increase in p-AMPK, PPARγ, UCP-1, and PGC-1α. Based on these results, it is inferred that RAL can induce thermogenesis in adipocytes, which is attributed to the decrease in lipid accumulation. There are various reports that the activation of AMPK regulates the expression of proteins related to lipolysis and thermogenesis (Gaidhu et al., 2009; Kim et al., 2020). In this study, the RAL-mediated increase of ATGL and HSL related to lipolysis, and PPARγ and UCP-1 related to thermogenesis were blocked by Compound C-mediated inhibition of AMPK. These results indicate that the regulation of lipolysis and thermogenesis-related protein expression by RAL is dependent on the activation of the AMPK signaling pathway. However, the lack of in vivo studies using animal models is a limitation of this study because this study was primarily performed in vitro. Therefore, additional in vivo studies using obese animal models are necessary for the clinical application of RAL in relation to its anti-obesity effects.
It has been reported that R. acicularis leaves contains various phenolic compounds such as flavonols, catechins, and ellagitannins, as well as non-phenolic compounds such as water-soluble polysaccharides and ascorbic acid (Olennikov et al., 2021). In addition, it has been reported that phenolic compounds, primarily epigallocatechin, catechin, Tellimagrandin II, Rugosin D, and miquelianin A, are the major constituents contained in R. acicularis leaves (Olennikov et al., 2021). Through various studies, it has been revealed that epigallocatechin and catechin among primary phenolic compounds contained in R. acicularis leaves exhibit anti-obesity activity (Kim et al., 2019; Rains et al., 2011). Furthermore, it has been reported that non-phenolic compounds such as polysaccharides and ascorbic acid also exhibit anti-obesity activity (Garcia-Diaz et al., 2014; Xu et al., 2015). Because the RAL used in this study is a water extract and these compounds such as epigallocatechin, catechin, polysaccharides, and ascorbic acid are water-soluble, the anti-obesity activity of RAL may be attributed to epigallocatechin, catechin, polysaccharides, and ascorbic acid. However, the lack of qualitative and quantitative analysis on functional components in this study is a limitation, and therefore, future research needs to include an exact component analysis study on RAL to identify which components exhibit anti-obesity activity.
In this study, we presented in vitro experimental evidence that RAL exhibits anti-obesity activity. It is suggested that RAL inhibits lipid accumulation through lipolysis via an increase in ATGL and HSL, and thermogenesis via an increase in PPARγ and UCP-1, through activation of AMPK in adipocytes (Fig. 4B). Based on these results, it is thought that RAL has the potential to be utilized as a potential candidate for the development of anti-obesity agents targeting lipolysis and thermogenesis.




