Introduction
Materials and Methods
Plant material
Surface sterilization
In vitro propagation conditions
Flow cytometry
Results and Discussion
Proliferation of adventitious bulblets under controlled media conditions
Examination of ploidy levels by flow cytometry
Introduction
Lily, a member belonging to the genus Lilium, is one of the most economically important and popular ornamental crops cultivated worldwide as cut flowers and outdoor garden plants in many countries (Xia et al., 2006). Consumer demand in the international flower market is often changing rapidly, and when new varieties are introduced to the market, old and unpopular varieties often disappear rapidly, despite their importance as genetic resources in the future. Also, the genus Lilium is facing a serious loss or genetic erosion due to weak immune system against pathogens, diseases, pests, adverse weather and other factors. Therefore, the development and supplementation of cryopreservation method, which is regarded as the only long-term method for the preservation of vegetative genetic resources, is an important strategy to respond rapidly to changing market demand and climate conditions (Halmagyi et al., 2004; Wang and Perl, 2006). After developing high- quality varieties of Lilium, since it is difficult to obtain disease- free bulbs, various in vitro tissue culture techniques have been used to establish mass propagation systems using bulb-scales (Joshi and Dhar, 2009) and apical meristems (Liu et al., 2011). In vitro tissue culture conservation is susceptible to contamination and somaclonal variation (Chen et al., 2011). Depending on the duration in vitro tissue culture in Lilium, challenges such as genetic variation, browning phenomenon, and reduced regeneration efficiency need to be addressed. In vitro cultures are associated with chromosomal variations, which are generally found in plants regenerated from long- term in vitro culture (Larkin and Scowcroft, 1981). Repeated subculture may reduce the regenerative efficiency (Wang et al., 2002), and increase the amounts of somatic variation (Scowcroft, 1984). Genetic changes can be reduced by decreasing the age of plant tissue by reducing the duration of the sub-culture period.
The effects of activated charcoal (AC) on morphogenesis have been reported in in vitro tissue cultures of previous studies (Fridborg and Erikson, 1975; Fridborg et al., 1978). It also facilitates generation of medium similar to natural soil environment following the addition of AC (Wang and Huang, 1976). However, Ahuja (1985) reported that although addition of AC to the medium increased shoot elongation and leaf size, the addition of AC alleviated the inhibition of shoot buds and reduced the number of shoot formations in Eucalyptus. Also, Yang et al. (1992) reported that the addition of AC to the medium increased shoot elongation and inhibited the number of shoots differentiated in Nicotiana tabacum. Based on the results of previous studies, this study investigated the positive or negative effects of AC on the growth and differentiation of bulblets in lily cultivars and increased the number of shoots from bulblets for use in cryopreservation.
Materials and Methods
Plant material
The lily accessions used in this study are TropicalPink (Lilium FA hybrid ‘FA97-2’ × L. Asiatic hybrid ‘Kokarde’) and GreenStar [(SilkyWhite × Sunray) × Bomi] cultivars of Lilium belonging to Liliaceae. The lily accessions were obtained from National Institute of Horticultural and Herbal Science (NIHHS) of the Rural Development Administration (RDA).
Surface sterilization
The plant materials used TropicalPink and GreenStar accessions to obtain disease-free bulblets from scales for cryopreservation in Lilium. Bulbs were washed to remove bacterials clump and residues under running tap water. Inner scales of the bulbs in these two cultivars were sterilized to the modified surface-sterilization as described previously by Askari et al. (2014) and Song et al. (2019). Scales detached from the bulbs were surface-sterilized for 20 min in 1.6% (w/v) sodium hypochlorite (NaClO). These scales were then rinsed for 3 min with sterile distilled water and 3 min with 0.03% NaClO on a clean bench. During the final step, we submerged the scales in 0.03% NaClO for 2 h instead of sterile distilled water. The inner scales were dried on the sterilized paper for 3 min.
In vitro propagation conditions
Plants during in vitro micropropagation were stored in the incubation room at 24 ± 1℃ under 16 h of photoperiod. The inner bulb scales were cultured and inverted on Murashige and Skoog (MS) medium containing 0.1% Plant Preservative Mixture (PPM, Apollo Scientific Limited, UK), which is a robust broad-spectrum biocide designed for use in plant tissue culture (Guri and Patel, 1998). The adventitious bulblets formed on the surface of inner scales were separated and cultured in MS medium, immediately. After bacterial examination on the Brain Heart Infusion (BHI, Difco, agar Becton Dickinson) agar medium plates (Song et al., 2019), non- contaminated bulblets were transferred to following media: MS basal medium supplemented with 1 g/L Charcoal (A medium), MS medium containing 0.3 ㎎/L indole-3-acetic acid (IAA) and 0.4 ㎎/L benzyl adenine (BA) hormone with (B Medium) or without Charcoal (C Medium) (Fig. 1).
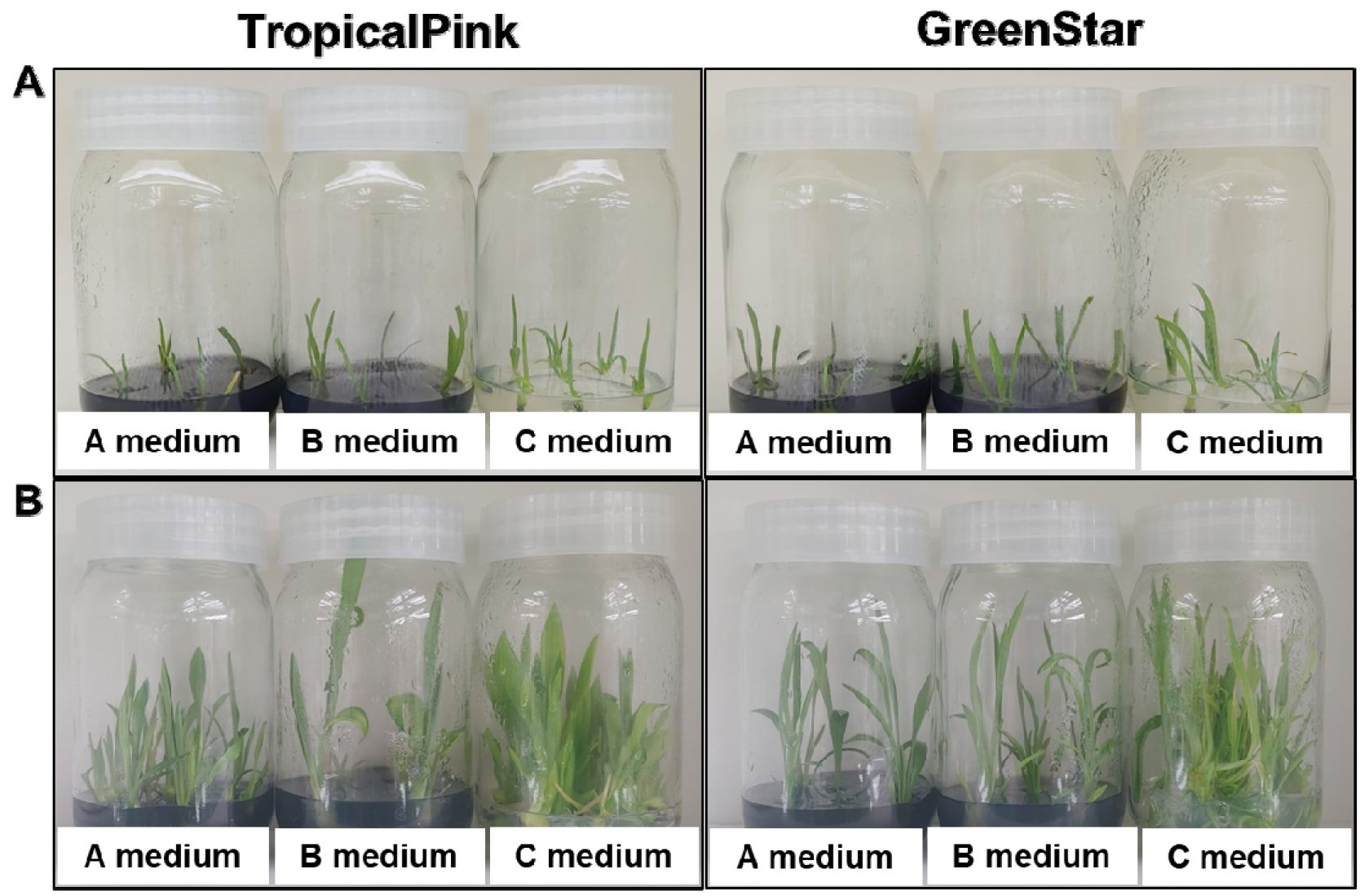
Fig. 1
Comparison of growth and propagation on three different media in two Lilium spp. The newly generated bulblets from inner scales were transferred to different media for 1 week (A) and the plants were propagated after 7 weeks of cultivation (B). Murashige and Skoog (MS) basal medium supplemented with 1 g/L Charcoal (A medium), MS medium containing 0.3 ㎎/L IAA and 0.4 ㎎/L BA hormone with (B Medium) or without Charcoal (C Medium).
Flow cytometry
The ploidy variations were analyzed by flow cytometry according to the procedure described by Naing et al. (2019). The plant sample, comprising about 20 ㎎ of leaf tissue, in a petridish containing 0.5 mL nuclei extraction buffer (Sysmex Partec GmbH, Germany) was chopped using a sharp razor blade. The chopped sample was filtered through 50 ㎛ nylon filter (CellTrics filter, Sysmex Partec GmbH, Germany) and the filtered solution was mixed with 2.0 mL of the staining buffer (Sysmex Partec GmbH, Germany). The prepared sample from each plant was analyzed to determine the ploidy variation using a CyFlow Ploidy Analyzer (Sysmex Partec GmbH, Germany).
Results and Discussion
The conventional and existing method of bulb-proliferation using in vitro tissue culture technology produces small bulbs directly by cultivating tissues such as scales for bulblet formation during in vitro micropropagation. Several bulbs can be produced via division of the new bulblet and re- cultivation. In vitro micropropagation is used to rapidly propagate cultivars, and results in the production of pathogen- free propagation sources (Hartmann et al., 2011). Although this method is a secure way for the vegetative propagation of lily, it is time-consuming and may induce increasing levels of somatic variation (Scowcroft, 1984), since the separation and regrowth of the bulblets may take months to reproduce via repeated subculture. Various studies focus on the factors contributing to efficient in vitro micropropagation, including the use of explants (Joshi et al., 2009), media conditions (Ozel et al., 2015), and callus induction (Youssef et al., 2019). Although some studies investigated the effects of AC on in vitro plant differentiation (Paek et al., 1998), additional studies are needed to confirm the effects of AC on the propagation of lilies. Therefore, in this study, we checked the effects of AC on in vitro plant propagation in lily cultivars and facilitated plantlets propagation by excluding of AC from the propagation medium for cryopreservation.
Proliferation of adventitious bulblets under controlled media conditions
Appropriate methods for in vitro propagation are important to increase the production rate and plant quality rapidly. This experiment was conducted to determine the most appropriate media conditions for propagation of adventitious bulblets of lily cultivars within the short period of time for cryopreservation. The multiplication method in this experiment is based on new bulblets formed from bulb-scales in two lily cultivars, TropicalPink and GreenStar. The adventitious bulblets formed from the inner scales were cultured on three different media containing MS basal medium supplemented with 1 g/L AC (A medium), MS medium containing 0.3 ㎎/L IAA and 0.4 ㎎/L BA hormone with AC (B medium) or without AC (C medium), respectively. After nearly seven weeks, the number of new plantlets propagated to small bulblets in the two media, A and B media, showed little variation (Fig. 1). However, the propagated plantlets belonging to TropicalPink and GreenStar cultivars in the C medium increased 1.5- and 1.8-fold, respectively. The number of multiple shoots increased about 5-fold in C medium compared to both A and B media. The number of propagated plantlets ranged from 5 to 6 and 4 to 6 with an average of 5 in TropicalPink and GreenStar cultivars, respectively (Table 1). However, the propagated plantlets were not rooted on C medium in both cultivars. New shoots formation and roots inhibition rather than plant growth was promoted in the medium including IAA 0.3+BA 0.4 without AC (C medium), while plant growth and development were induced by absorbing hormones, especially BA, in the B medium supplemented with AC (Fig. 1). Thus, the formation of multiple shoots was induced by the accumulation of plant hormones in the bulblets on C medium containing IAA and BA by excluding AC. The most crucial feature of adding AC to the culture media is decline in concentration of the added hormones, naphthaleneacetic acid (NAA) and BA, in the medium due to the absorption of the growth regulating substance by AC (Steimitz and Yahel, 1982). Similar results were reported that new shoots are induced from plantlets laterally due to excessive levels of plant hormones such as auxin and cytokinin (Amasino and Miller, 1982) and by another study in Nicotiana tabacum (Yang et al., 1992). Most of the reports confirmed the positive effects of AC in medium promoting growth and development of plant tissues (Ebert et al., 1993; Fridborg et al., 1978). The addition of AC to the plant tissue culture medium induces absorption of growth inhibitors secreted from culture tissues, inhibits the growth of calli, and root formation of somatic embryogenesis (George and Sherrington, 1984). However, AC induced negative results were also reported in some micropropagation. The addition of AC to overcome phenolic exudation in the medium reduced the number of shoots due to the adsorption of hormones needed for plant growth in Gymnema sylvestre micropropagation (Komalavalli and Rao, 2000). Further, the addition of AC to the medium increased shoot elongation and leaf size, but suppressed the shoot formation and differentiation in Eucalyptus (Ahuja, 1985), N. tabacum (Yang et al., 1992), and cashew (Anacardium occidentale) (Boggetti et al., 1999).
Table 1
Propagation of small bulbs induced from inner scales after transfer to the different media in two lily cultivars
Based on various aspects of the effects of AC on in vitro micropropagation, we induced multiple shoots of the adventitious bulblets on the C medium containing the plant hormones by excluding AC. The proliferation technique used in this study reduced the long-term culture of plant by reducing repeated subculture, and provided basic information for multiple shoot formation from adventitious bulblets in lily cultivars for use in cryopreservation.
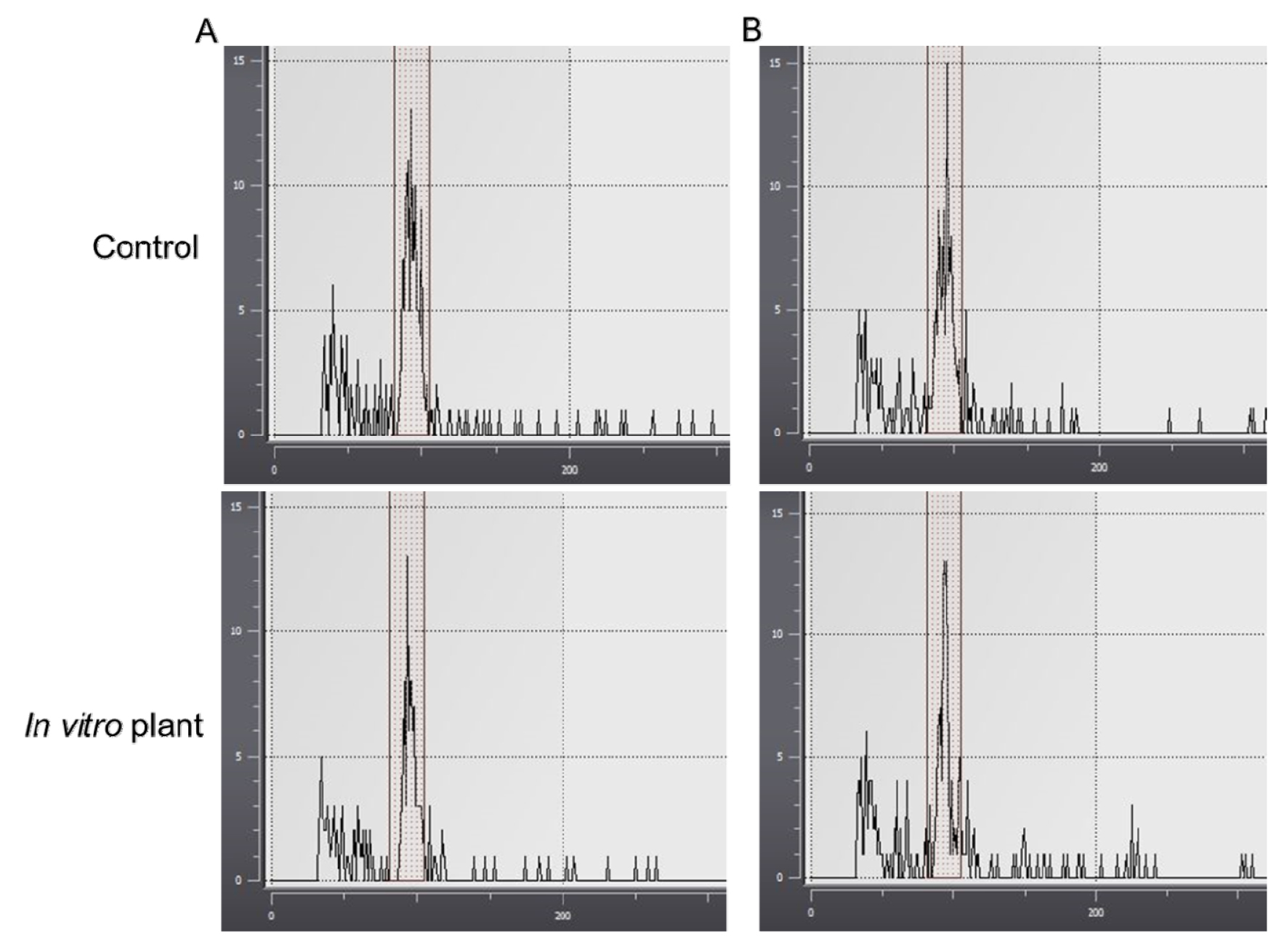
Examination of ploidy levels by flow cytometry
The ploidy levels of plants grown generally in the greenhouse (control) and in vitro propagated plants of two different lily cultivars, TropicalPink and GreenStar, were measured by flow cytometry. As a result of the ploidy test, the ploidy levels between the control and in vitro plant indicated no difference in the number of chromosomes or genetic variation (Fig. 2). Therefore, the in vitro propagated plants of the two cultivars are genetically similar to the control plants grown in the greenhouse by reducing the duration of in vitro propagation and the repeat subculture in this study.