Introduction
Materials and Methods
Plant material
General information
Moisture content
Germination test
Cold stratification
Alternating stratification temperatures
Improving Germination Rate by Application of GAs
Data analysis and statistical processing
Results
General information and germination test
Cold stratification
Stratification at temperature sequences
Improving Germination Rate by GA Treatment
Discussion
Introduction
Chelidonium majus L. subsp. asiaticum H. Hara is biennial herb of the Papaveraceae family widely distributed across Eastern Asia (KBIS, 2021; Maji and Banerji, 2015). Plants grow 30-80 ㎝ high, with alternate leaves, and the yellow umbel flowers bloom between May and August (KBIS, 2021). An orange-yellow latex oozes out from the stem, from which the Korean name of C. majus L. subsp. asiaticum H. Hara was derived (Maji and Banerji, 2015). In traditional oriental medicine, the aboveground part of C. majus L. subsp. asiaticum H. Hara is called “baekgulchae,” which is used for pain relief and to treat diuresis, hepatitis, and gastric ulcers (Jung et al., 2011). In addition, C. majus L. subsp. asiaticum H. Hara contains various alkaloid compounds with antibacterial, antioxidant, and anti-inflammatory effects (Kim et al., 2000). Despite its high value as a natural source of potential pharmaceutical active principles, there are no reports available on seed propagation to ensure a sufficient number of seedlings to develop new medicines.
As it does not require intensive labor, seed propagation is the simplest commercial method used to propagation numerous plant species of human interest (Abbad et al., 2011). However, seed propagation is affected by various abiotic factors, such as temperature, light, and moisture during dispersal and germination (Haeussler and Tappeiner, 1993), which is known to occur under appropriate conditions for plant growth. Seeds may undergo various types of dormancy depending on the environment. Dormancy is considered as an important strategy for survival after seed maturation (Baskin and Baskin, 2014) and is essential to understand dormancy and dormancy types for successful seed propagation.
Dormancy is a mechanism that inhibits seed germination under unfavorable environmental conditions (Hilhorst, 1995). Nikolaeva (2004) devised a dormancy classification system because this survival mechanism is determined by the morphological and physiological characteristics of seeds. Subsequently and based on this system Baskin and Baskin (2004) divided seed dormancy into five classes including: physiological dormancy (PD) due to inhibition of germination from inside seeds; morphological dormancy (MD) due to embryo immaturity; morphophysiological dormancy (MPD), in which both morphological and physiological dormancy occur simultaneously; physical dormancy (PY) due to the impermeability of the seed or fruit coat; and combined dormancy (PY+PD), in which both physical and physiological dormancy occur simultaneously. Dormancy can be further subdivided into various levels and types (Ryu et al., 2017).
Plant seeds that are native to temperate regions, such as Adonis amurensis, Iris laevigataand and Jeffersonia dubia have immature embryos during their dispersal (Ko et al., 2022; Lee et al., 2012; Martin, 1946). Seeds of Papaveraceae, such as Papaver argemone, P. dubium subsp. dubium (Karlsson and Milberg, 2007), and P. rhoeas (Baskin et al., 2002), can exhibit MPD (Baskin and Baskin, 2014; Harper and Van Buren, 2004), including C. majus L. subsp. asiaticum H. Hara (Grime et al., 1981; Martinkova and Honěk, 1997). Thus, C. majus L. subsp. asiaticum H. Hara used in this study presumably exhibits MPD. However, there is currently no information on the levels and types of seed dormancy in C. majus L. subsp. asiaticum H. Hara, or methods of overcoming such dormancy.
In general, MPD in seeds can be broken by warm and/or cold stratification or gibberellic acid (GA) treatment. Stratification is a treatment of controlling dormancy and germination timing by presenting the temperature required for seed germination (Baskin and Baskin, 2014). GA is a hormone that regulates seed dormancy, embryo development, and germination and is used to break dormancy of various types, including MPD. The responses of seeds to GA treatment vary according to the degree of dormancy, including non-deep, intermediate, and deep MPD, and the germination-promoting effects of GA decreases as the degree of PD increases (Kim and Na, 2021; Kim et al., 2022; Mamut et al., 2014). In addition, MPD is classified into two subclasses and nine levels according to the required stratification temperature and the GA substitution ability.
An effective propagation system is required to enable further research on the seed dormancy of Korean C. majus L. subsp. asiaticum H. Hara. Therefore, this study investigated the levels of dormancy in C. majus L. subsp. asiaticum H. Hara seeds as well as the dormancy-breaking conditions. In addition, a dormancy-breaking treatment was established based on stratification and GA treatment. The results of the present study could provide basic data that could facilitate C. majus L. subsp. asiaticum H. Hara seed propagation and commercial cultivation of the medicinal plant.
Materials and Methods
Plant material
The C. majus L. subsp. asiaticum H. Hara seeds used in this study were collected in Bonghwa-gun region, Gyeongsangbuk- do, in June 2019 and were distributed to the Baekdudaegan Natuonal Arboretum. After cleaning, the collected seeds were placed in a plastic bag and sealed. Seeds were then dried at room temperature (25 ± 1.0℃) and then used for the experiment 8 months later.
General information
Observations of the outer and inner structure, weight, size, viability, and moisture content of seeds were investigated. To observe the shape of the embryo after sowing and just before germination, the transverse section of the seed was obtained using a razor blade (Stainless blade, Dorco Co. Ltd., Seoul, Korea), and the inner structure was observed with a stereomicroscope (SZ61, Olympus, Tokyo, Japan). Seed size was measured using a vernier caliper (Digimatic caliper, Mitutoyo, Kawasaki, Japan) (n = 15), and the weight was measured using a microbalance (AS 220.R2, Radwag Uk Ltd., Radom, Poland). The weight of 1,000 seeds was measured in four replicates. Viability of the seeds was investigated by immersion in 20℃ distilled water for 72 h followed by 24 h incubation in 1% tetrazolium solution (triphenyl tetrazolium chloride, Sigma- Aldrich, St. Louis, MO, USA) at 30℃ in a seed germination chamber.
Moisture content
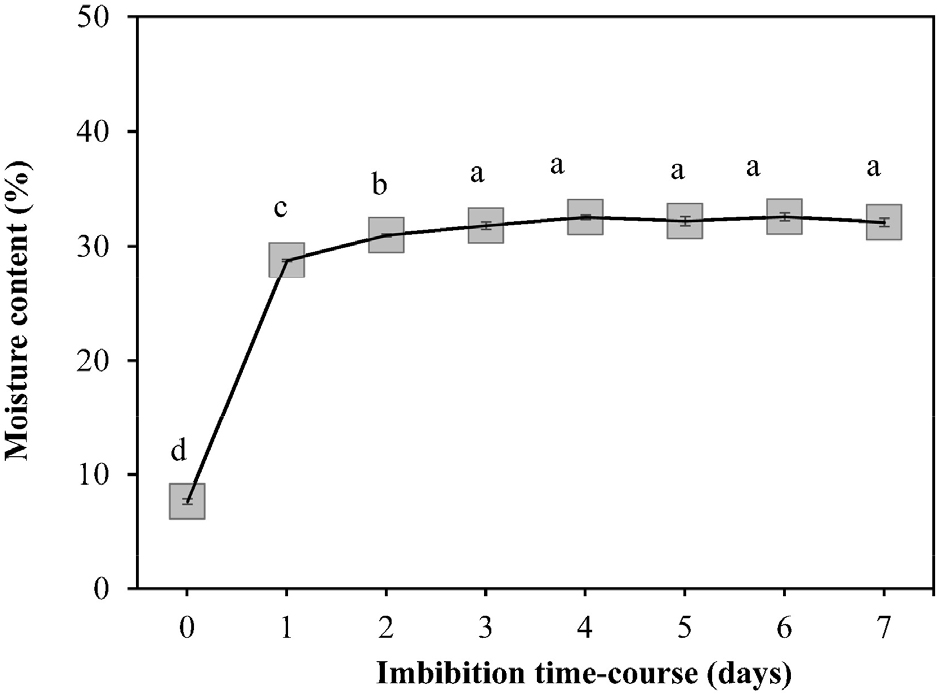
To measure the moisture content, the weight of 100 seeds was measured (n = 4) before immersion. Then, seeds were placed in a 15 mL conical tube (50015, SPL Life Science Co. Ltd., Pocheon, Korea) and stored at 4℃. After 24 h of immersion in distilled water, the weight of the seeds was measured using a microbalance. Before measurement, seeds were blotted dry with a paper towel (Drycell hand towel, Yuhan Kimberly, Ltd., Seoul, Korea). After measurement, seeds were immersed and stored at 4℃, and the measurement was continued for seven consecutive days. Seeds were then oven-dried at 60℃ for 48 h to measure dry weight and determine the initial moisture content.
Germination test
Seeds used for the germination test were disinfected for 10 min with 1% sodium hypochlorite (Yuhanrox, Yuhanclorox Co., Ltd., Seoul, Korea) and rinsed with water three times before initiation of the experiment. Seeds were placed in a Petri dish (10010, SPL Life Science Co. Ltd., Pocheon, Korea) with two sheets of filter paper (qualitative filter paper grade 1, 90 ㎜, Whatman Co. Ltd., Maidstone, England), and moistened with 4 mL of distilled water. Germination experiments were performed according to the procedure followed by Baskin and Baskin (Baskin and Baskin, 2003). The germination test was conducted for 30 days in a growth chamber under a 25/15℃ day/night temperature regime, and a 16/8 h light/dark regime (30 µmol/m2/s PPFD, White-LED), or under conditions of continuous darkness, in which case, aluminum foil was used to completely block light. Distilled water was added as required during the experiment to prevent the filter paper from drying out. In the light/dark cycle condition, the germination rate was checked daily at the same time for 30 days, while only the final germination rate was investigated after 30 days under conditions of continuous darkness. Each seed was considered to have germinated when the emerged radicle reached at least 1 ㎜ of length.
Cold stratification
The cold stratification (weeks at winter temperature) environment was prepared by filling a plastic box with sterilized pearlite (New Pershine No. 2, GFC. Co., Ltd., Hongcheon, Korea), where filter bags containing the seeds were placed. The humidity of the perlite was 96.60 ± 0.589%. The box was placed in a chamber at 4℃ for 12 weeks, and seeds were removed and sown every two weeks. Subsequently, the germination test was conducted under the same conditions as the basic germination test described above, and embryo growth was observed using a stereomicroscope before sowing.
Alternating stratification temperatures
To simulate the natural environment of the habitat of seeds, temperature treatments prior to germination tests were set as follows: warm stratification (S, summer temperature) was applied in a chamber set at 25/15℃ day/night temperature regime and a 16/8 h photoperiod (30 µmol/m2/s PPFD, White- LED) for 12 weeks, followed by intermediate temperature stratification (A, autumn temperature) in a chamber set at 15/ 10℃ day/night temperature regime and a 16/8 h photoperiod (30 µmol/m2/s PPFD, White-LED) for four or eight weeks. Then, stratification and germination tests were conducted in the same way as described previously.
Improving Germination Rate by Application of GAs
The improvement of germination rate by gibberellin treatment was tested on seeds subjected to the alternating-temperature stratification treatment, which had the best dormancy-breaking effect. Before sowing, seeds stratified with treatment S12-A4 were immersed in 500 ㎎/L GA3 (Cas No. 77-06-5, Sigma- Aldrich, St. Louis, MO, USA) or GA4+7 (Cas No. 468-44-0/ 510-75-8, KisanBio, Seoul, Korea) at 4℃ in the dark for 24 h. Germination testing and observation were performed as in the previous experiments.
Data analysis and statistical processing
Germination and stratification experiments were performed with four replications with 100 seeds each. Seed images were obtained with a CMOS camera (eXcope F630, Dixi Sci., Daejeon, Korea) attached to a stereomicroscope and the eXcope software (ver. 3.7.12277) was used to capture the images. Mean and standard error of all collected data were obtained, and ANOVA was performed using the Statistical Analysis Software (SAS) (ver. 9.4, SAS Institute Inc., Cary, NC, USA) to evaluate the differences among treatments, followed by post-hoc Duncan's multiple range test (DMRT) to separate significantly different (P < 0.05) means.
Results
General information and germination test
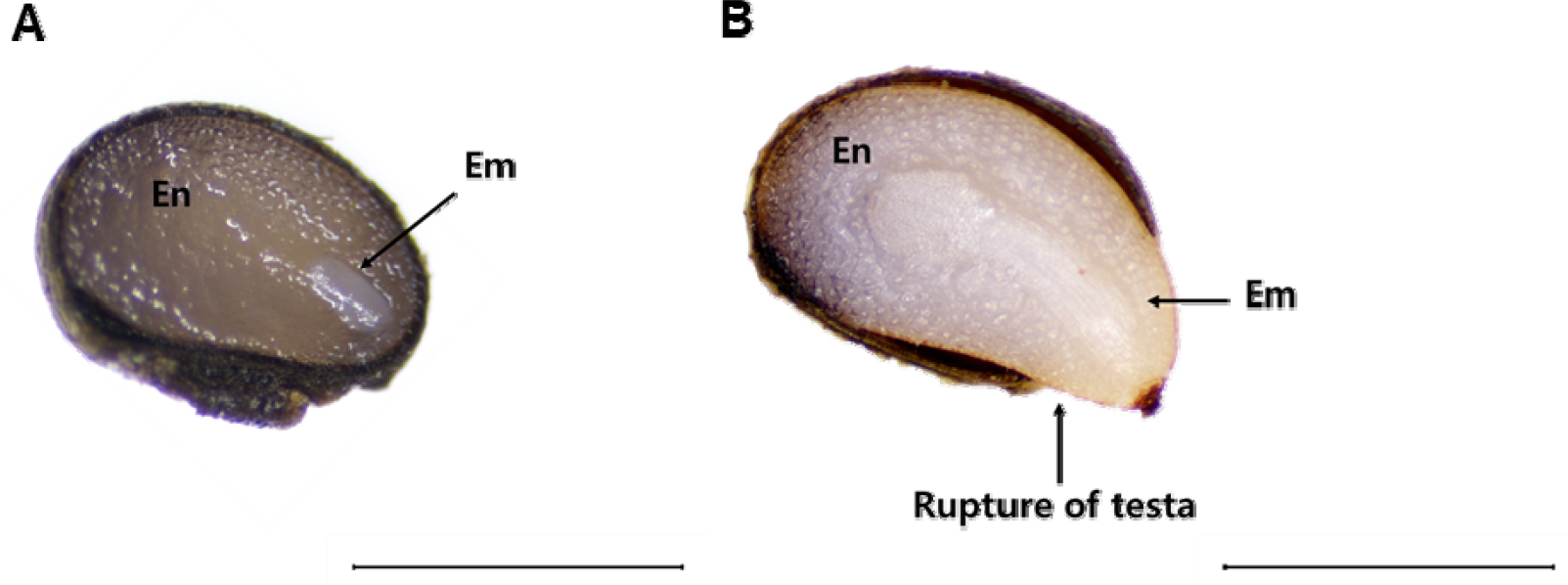
Seeds of C. majus L. subsp. asiaticum H. Hara were blackish- brown in color and had elaiosomes; fleshy lipid and protein- rich structures attached to their surface. Mean of longitudinal and transverse sections of the seeds were 1.24 ± 0.018 ㎜ and 0.83 ± 0.013 ㎜, respectively, and their mean weight was 0.57 ± 0.012 g. Seed viability was estimated to be 79% as per the tetrazolium test (Table 1). White embryos and transparent endosperms were observed in the longitudinal sections of the seeds. The embryo length to seed length ratio (E:S) after dispersal and just before germination was 0.21 ± 0.010 and 0.73 ± 0.042, respectively. This indicates that the embryo before germination is morphologically immature and steadily grows until just before germination, as seen in Fig. 1. Seed moisture content increased rapidly over the first 24 h of imbibition and plateaued (31.82 ± 0.320%) on the third day of imbibition (Fig. 2). Preliminary experiments showed 0.80 ± 0.750% and 5.50 ± 1.560% germination of dormant seeds under a light-dark regime and continuous darkness, respectively (Table 1).
Table 1.
Basic characterization of C. majus L. subsp. asiaticum H. Hara seeds
| Scientific name | Sizez (㎜) |
Weighty (g) |
Viability (%) | Germinationx (%) | ||
| Length | Width | Light / dark | Dark | |||
| Chelidonium majus L. subsp. asiaticum H. Hara | 1.24 ± 0.018 | 0.83 ± 0.013 | 0.57 ± 0.012 | 79 | 0.80 ± 0.750 | 5.50 ± 1.560 |
Cold stratification
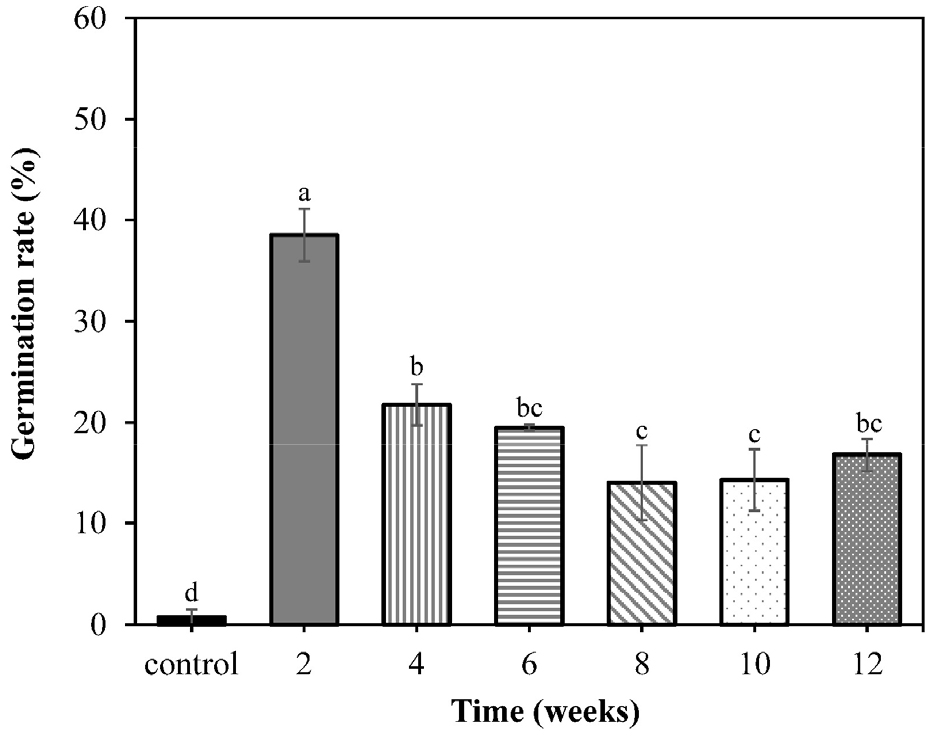
The germination rate of untreated seeds (without stratification) was 0.8 ± 0.75%, while the maximum germination rate (38.5 ± 2.60%) was confirmed in the seed groups subjected to the 2 weeks cold stratification treatment (Fig. 3). However, germination tended to decrease gradually as the duration of the cold- stratification treatment increased, with the 6 weeks treatment showing the lowest percentage.
Stratification at temperature sequences
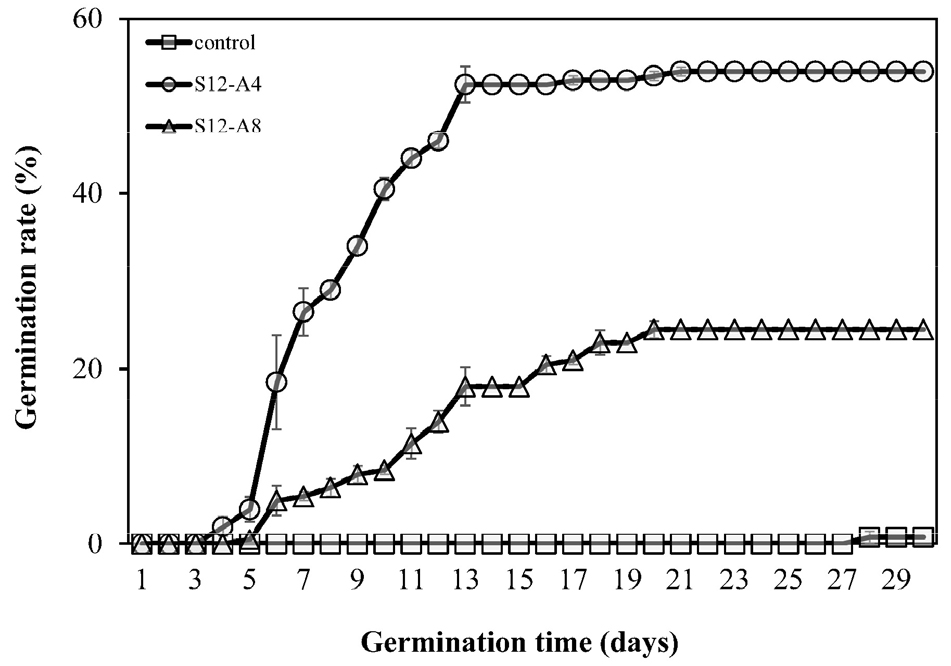
The applying alternating temperature stratification to simulate the ecological environment of the natural habitat and germination period of C. majus L. subsp. asiaticum H. Hara resulted in the highest (54.0 ± 5.83%) final germination rate in the S12-A4 treatment group, with a relatively short “autumn” period. In contrast, the germination rate in the S12-A8 treatment group, with a long “autumn period,” was only 24.5 ± 7.32% (Fig. 4), indicating that the germination rate of C. majus L. subsp. asiaticum H. Hara seeds tends to decrease with the increasing duration of the “autumn” period.
Improving Germination Rate by GA Treatment
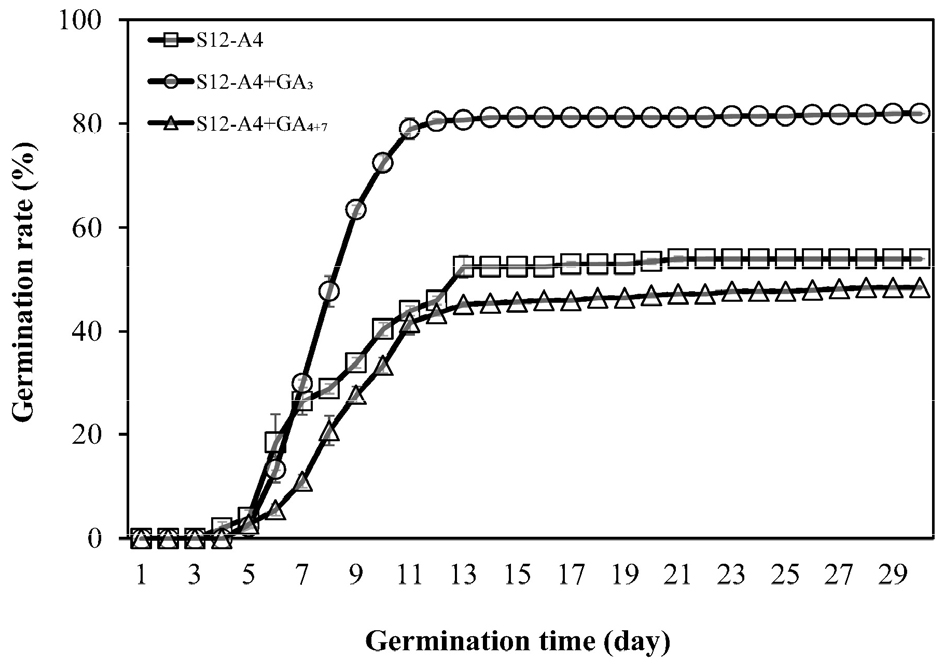
In this experiment, after the alternating-temperature stratification treatment (S12-A4), which exhibited the highest final germination rate, each seed was treated with GA3 or GA4+7. The germination rate in the GA3 treatment group (82.0 ± 2.55%) was improved by 28%, relative to alternate-temperature stratification treatment alone (Fig. 5). Conversely, the GA4+7 treatment group showed a lower germination rate (48.5 ± 3.62%) than the GA4+7-untreated seeds in the alternate-temperature stratification treatment group.
Discussion
C. majus L. subsp. asiaticum H. Hara seeds have small, oval, rudimentary immature embryos (Martin, 1946). Dried seeds showed a very low germination rate at 30 days after harvest. According to Nikolaeva (2004), seeds germinating within 30 days from harvest and showing visible embryo growth through a germination test are characterized by MD, while those that do not germinate most likely exhibit MPD. Furthermore, as moisture absorption in these seeds was 20% higher compared to the initial moisture content, impermeability due to the external structure of the seed was not confirmed. MPD is the class of dormancy that combines both MD and PD and can be subdivided into two subclasses and nine levels.
In this study, embryo growth in C. majus L. subsp. asiaticum H. Hara seeds were not observed during the cold stratification period (data not shown), but a relatively high germination rate (38.5%) was recorded when compared to the non-stratified seeds. Arabidopsis thaliana seeds reportedly show PD, and their dormancy is known to break when cold- stratified for a short period of time (Fedi et al., 2017). However, the dormancy of C. majus L. subsp. asiaticum H. Hara seeds were partially broken by cold stratification. In this case, dormancy was not broken by the winter temperature, as evidenced by the significantly lower germination rate observed as compared to the measured viability (79%), but the germination rate decreased as the duration of cold stratification increased, similar to that reported for Ambrosia artemisiifolia (Willemsen, 1975).
Similar to C. majus L. subsp. asiaticum H. Hara, some of the winter annual seeds that germinate in autumn and grow through the winter into the following year are known to exhibit conditional dormancy under late autumn and winter temperatures (Baskin and Baskin, 1981; 1985; Froud-Williams et al., 1984; Roberts and Neilson, 1982). This suggests that C. majus L. subsp. asiaticum H. Hara may enters conditional dormancy when the period of unfavorable winter temperatures extends. Some of the seeds that did not germinate in the cold stratification experiment showed another type of dormancy. However, this phenomenon warrants further study to determine the methods to overcome this type of dormancy. Some plant species require alternating temperature stratification between winter and summer to overcome MPD (Finch-Savage and Leubner-Metzger, 2006). In the alternating-temperature stratification experiment, the germination rate of S12-A4 was higher than that of cold stratification, but the germination rate of S12-A8 was relatively low. Similar to the results observed for cold stratification, in this case, the germination rate also tended to decrease as the intermediate temperature period which was at a relatively low temperature compared to summer temperatures extended. Thus, based on the above experiments, C. majus L. subsp. asiaticum H. Hara exhibited two types of dormancy in this study.
Xanthium exhibits two types of dormancies (Bewley et al., 2012), deep and shallow, whereas Chenopodium album exhibits four types (Karssen, 1976). Therefore, having two or more types of dormancies is a mechanism to control the species population by varying the temporal distribution at which dormancy is broken to prevent competition among individuals of the same species and thus, increase the survival rate. C. majus L. subsp. asiaticum H. Hara blooms between May and August (KBIS, 2021). Such a long flowering period suggests that the initiation time for germination may be different. The first germination of the year starts in early spring, and flowering and fruiting occur before summer, while subsequent germination starts in late summer and flowering and fruiting in early autumn. In this study, the dormancy of some seeds was broken by a short cold- stratification treatment as it occurs in nature with seeds borne in fruit in the autumn, which germinate the following year. Meanwhile, the remaining seeds, whose dormancy was broken by cold stratification, are believed to germinate in the autumn. Therefore, C. majus L. subsp. asiaticum H. Hara seeds have two levels of MPD: intermediate simple and non-deep simple.
In addition, the seeds whose intermediate simple dormancy which is known to be broken by winter temperature was broken by the alternating-temperature stratification treatment, had their non-deep simple dormancy effectively terminated when GA3 was subsequently applied, resulting in a germination rate (82.0%) comparable to the measured vitality of the seed. Therefore, non-deep simple MPD levels can be relieved by GA3. GA is involved in several stages of plant development, including stem and root elongation, fruit development, and flowering (Hedden and Thomas, 2012). In particular, GA is known to break dormancy by offsetting the effect of ABA-induced dormancy (Kucera et al., 2005). After alternate temperature stratification, the highest germination rate was shown in GA3 (82%). GA3 is known to break dormancy by being synthesized during cold stratification in some plants. Thus, for example, germination of A. thaliana seeds was reportedly promoted by the upregulation of GA biosynthesis-related genes, such as GA3ox1, under winter temperatures (Yamauchi et al., 2004). In the seeds of Zanthoxylum nitidum, whose intermediate physiological dormancy was broken through cold stratification and GA treatment, the difference in the proteins expressed in the seeds between the treatments was also investigated (Lu et al., 2018). When Arbutus unedo L. seeds were treated with 1000 ㎎/L of GA3, the germination rate was 63%, which was similar to that of the seeds treated with cold stratification (67.75%) for 2 months (Smiris et al., 2006). Therefore, it was confirmed that GA3 treatment effectively improved the germination rate by replacing the winter temperature stratification and breaking the dormancy of C. majus L. subsp. asiaticum H. Hara seeds. In contrast, GA4+7 showed a lower germination rate (49%) than that of the untreated group (54%). The type and appropriate concentration of GA for improving the germination rate is believed to be species-dependent (Bewley and Black, 1982).
In conclusion, GA3 treatment after S12-A4 alternating- temperature stratification treatment effectively broke both levels of MPD in seeds of C. majus L. subsp. asiaticum H. Hara native to Korea. The two levels of MPD observed were non-deep simple and intermediate simple MPD are considered to exhibit polymorphism in dormancy. Non-deep simple MPD was ended by exposure to winter temperatures, while intermediate simple MPD was ended by exposure to summer and autumn temperatures. For both the types of dormancies, cold stratification effectively substituted for GA3, while GA3 treatment applied after alternate-temperature stratification can be expected to result in a high germination rate, similar to the viability rate of seeds. Therefore, we conclude that this is an efficient seed propagation method that can be used for pharmaceutical or industrial mass propagation of C. majus L. subsp. asiaticum H. Hara.