Introduction
Materials and Methods
Materials
Preparation of BB extract
RAW264.7 macrophage culture
Cell viability determination
Cytokine assay
PGE2 assay
Isolation of cytosol and nuclear fractions and western blot analysis
Caspase-1 activity determination
Statistical analysis
Results
Effects of BB on cell viability and inflammatory cytokines levels in LPS- activated RAW264.7cells
Effects of BB on COX-2 expression and PGE2 production in LPS- activated RAW264.7 cells
Effects of BB on IκB-α degradation and NF-κB translocation in LPS- activated RAW264.7 cells
Effect of BB on caspase-1 activation in LPS- activated RAW264.7 cells
Discussion
Introduction
Inflammation is a protective response of the immune system contributing to the host defense against external stimuli including viruses, bacteria, and toxic compounds (Zhang and Wang, 2014). However, excessive or prolonged inflammatory response can lead to various chronic diseases including rheumatoid arthritis, hepatitis, and atherosclerosis (Du et al., 2015). In inflammatory condition, activated macrophages release interleukin (IL)-6, tumor necrosis factor (TNF)-α, leading to tissue damage (Liu et al., 2014; Martinez et al., 2008). Increased prostaglandins (PGs) and cyclooxygenase (COX)-2 contributes to the pathophysiological progression of inflammatory disorders (Fiorucci et al., 2003). Especially, PGE2 induced by COX-2, causes to the swelling associated with inflammation and pain (Mann et al., 2005). Therefore, the down-regulation of inflammatory mediators could be beneficial in treating of inflammation-related disorders.
Nuclear factor-κB (NF-κB) is a transcription factor that modulates the expression of inflammation-related genes (Ghosh and Hayden, 2008). In unstimulated cells, heterodimer of NF-κB (p50/p65) exists in a latent inactive form complexed to an inhibitor protein, IκB-α. Under inflammation, IκB-α is phosphorylated and then degraded to induce its dissociation of NF-κB dimers. The free NF-κB migrates to the nucleus and induces expression of numerous inflammatory factors (Majdalawieh and Ro, 2010). Studies have shown that NF-κB activity is increased in patients with inflammatory diseases. Caspase-1, inflammatory caspases, contribute to a variety of biological functions involved in apoptosis and inflammation (Broz et al., 2010). There is emerging evidence that caspase-1 regulates the inflammatory genes expression and deficiency of caspase-1 reduce the inflammation (Han et al., 2017). Dexamethasone, commonly used as an anti-inflammatory, modulate inflammation-mediated lung injury through suppression of caspase-1 activity in lung tissues (Guan et al., 2020). Therefore, alleviation of NF-κB pathway and caspase-1 activation is novel therapeutic targets for inflammatory disorder.
Plants as diverse as Myriophyllum spicatum L. are known as natural sources of antioxidants such as seeds, fruit herbs, and vegetables (Kim et al., 2023). Blueberry (BB), fruit of Vacciniumi genus, is a rich source of vitamin, flavonoids and phenolic acids (Chen et al., 2010). It has been hailed as an antioxidant superfood. BB has a variety of pharmacological actions, including antioxidant, anti-obesity and anti-angiogenesis effects (Skrovankova et al., 2015; Wu et al., 2013). In this regard, the aim of this study is to clarify the anti-inflammatory mechanism of BB in lipopolysaccharide (LPS)-activated RAW264.7 macrophage.
Materials and Methods
Materials
3-(4,5-dimethylthiazol-2-yl)-diphenyl-tetrazoliumbromide (MTT), dimethyl sulfoxide (DMSO), LPS, avidin peroxidase (AP), Phosphate-buffer saline (PBS) and other reagents were supplied from Sigma Aldrich (St. Louis, MO, USA). Bicinchoninic acid (BCA) and enhanced chemiluminescence (ECL) kit were obtained by Thermo Fisher Scientific Inc. (Rockford, IL, USA). The mouse ELSIA kits for IL-6 and TNF-a were obtained from BD Biosciences (San Jose, CA, USA). COX-2, NF-kB and IκB-α antibodies were procured by Santa Cruz Biotechnology (Santa Cruz, CA, USA). Caspase-1 assay kit was obtained from R&D System (Minneapolis, MN, USA).
Preparation of BB extract
BB was procured from Jeon-ju Agricultural Wholesale Market (Jeonju, Jeonbuk-do, Korea). The fruit freeze driedof BB (100 g) was dipped in 70% ethanol (1 L) and maintained for 24 h. Subsequently, the extract was evaporated under vacuum rotary evaporator (Eyela, Japan). The extract (yield, 10.2%) was lyophilized and then frozen and kept at 4℃ until analysis. The BB extract was dissolved in PBS and filtered through a 0.22 ㎜ syringe filter.
RAW264.7 macrophage culture
Cells were maintained in Dulbeco’s Modified Eagle’s Media containing with 10% Fetal Bovine Serum, streptomycin (100 ㎎/mL) and penicillin (100 IU/mL) in 5% CO2 atmosphere at 37℃.
Cell viability determination
To evaluate the cell viability by BB, the MTT assay was conducted. Cells were treated with various concentrations of BB extract (0.01, 0.5, and 1 ㎎/mL) for 24 h, and 50 μL of MTT solution (5 ㎎/mL) were mixed. After incubation for 4 h, DMSO was added to dissolve the crystallized formazan, and the optical density was quantified at 580 ㎚ using a microplate reader (Molecular Devices, USA).
Cytokine assay
Cells were treated with BB (0.01 to 1 ㎎/mL) for 1 h and treated with LPS (1 μg/mL) and for 12 h. The levels of IL-6 and TNF-a from cell were analyzed by using ELISA kits, adhering to the manufacturer’s protocol. Briefly, plates were coated with IL-6 and TNF-a monoclonal Abs and then incubated overnight. After washing, standards of IL-6 and TNF-a or sample were added. After washing, the plate was reacted with biotinylated IL-6 and TNF-a Abs followed by incubation for 2 h. After then, AP and ABTS was sequentially exposed. The optical density was determined at 405 ㎚. Concentration of IL-6 and TNF-a was determined by comparison with a standard curve.
PGE2 assay
Cells (3 × 105 cells/well) were treated h with various dose of BB (0.01 to 1 ㎎/mL) and incubated with LPS for 12 h. The PGE2 content from cell was measured using PGE2 enzyme immune assay. Briefly, the sample or PGE2 standards were added to coated plates of 96-well for 2 h. After washed, 50 μL of PGE2 conjugate Ab were incubated. Washing with washing buffer and reacted with 100 μL of substrate solution and added with 50 μL of stop solution. Optical density was determined at 450 ㎚. PGE2 concentration was assessed by comparison with a standard curve.
Isolation of cytosol and nuclear fractions and western blot analysis
Cells (5 × 106 cells/mL) were treated with BB, followed by LPS treatment for 2 h. The cells were washed with PBS, collected, and cytosol and nuclear fractions were isolated with NE-PER Nuclear Extraction reagents following the manufacturer’s protocol. After protein quantification using BCA, the lysed protein was subjected to sodium dodecyl sulfate-PAGE for separation and then electrotransfered to PVDF membrane. The membrane was then blocked with 5% nonfat milk, washed and reacted with primary Abs overnight and then secondary Abs for 2 h. After washing, ECL detection reagent (Thermo Fisher Scientific Inc., USA) was utilized to develop the protein bands.
Caspase-1 activity determination
Caspase-1 colorimetric assay kit was conducted for quantification of caspase-1 activity, adhering to manufacturer’s protocols. After protein quantification using BCA, the sample was incubated with 50 μL of reaction buffer and 5 μL of substrate for 2 h. Color development was assessed at 405 ㎚ using a microplate reader.
Statistical analysis
Results were shown as mean ± standard deviation (S.D). Independent t-test and ANOVA analysis of variance was used to assess the statistical significance. Values of P < 0.05 was considered significant.
Results
Effects of BB on cell viability and inflammatory cytokines levels in LPS- activated RAW264.7cells
To identify the effects of BB on cell viability, cells incubated with different concentrations of BB (0.01, 0.5 and 1 ㎎/mL) for 24 h and conducted by MTT assay. The results shows that cell viability was maintained following treatment with different concentrations of BB and BB did not show cell cytotoxicity against macrophage cells even up to the concentration of 0.01 to 1 ㎎/mL (Fig. 1A). Next, we assessed whether BB can attenuate the LPS- mediated inflammatory cytokine levels in RAW 264.7 macrophage. The cells were incubated with various concentrations of BB (0.01, 0.5 and 1 ㎎/mL) and treated with LPS for 12 h. Supernatant was collected and analyzed for IL-6 and TNF-a production by ELISA kits. As shown in Fig. 1B and C, LPS alone enhanced IL-6 and TNF-a levels, whereas BB markedly suppressed LPS-induced IL-6 and TNF-a levels in a concentration-dependent manner. The inhibition rates of IL-6 and TNF-a secretion by BB (1 ㎎/ mL) were approximately 36.4% (p < 0.05), and 37.7% (p < 0.05), respectively.
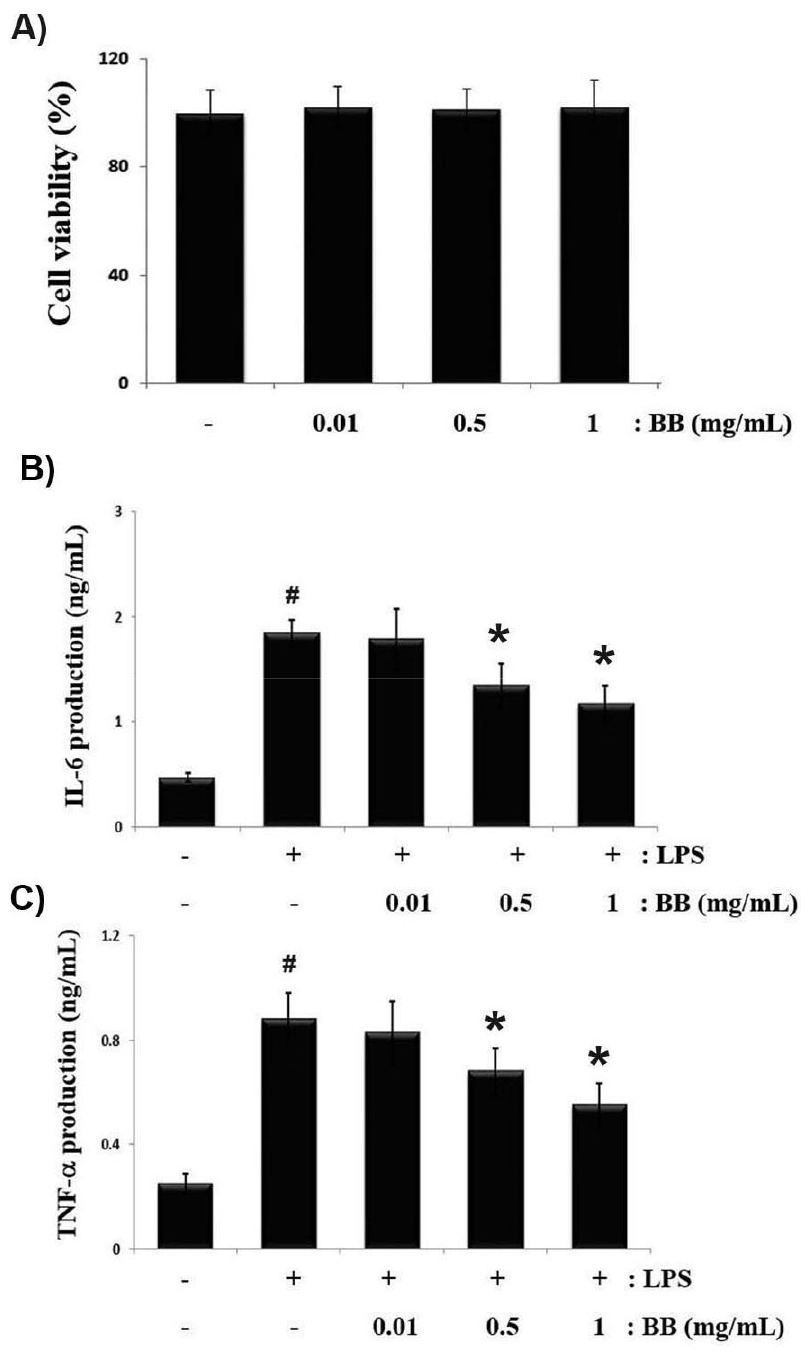
Fig. 1.
Inhibitory effects of BB on LPS-activated IL-6 and TNF-α production in RAW264.7 macrophages. Cells (3 × 105 cells/mL) were treated for 1 h with various dose of BB (0.01, 0.5, and 1 ㎎/mL), and incubated with LPS (1 ㎎/mL) for 12 h. (A) Cell viability was measured using MTT assay to define the cell toxicity of BB. (B,C) ELISA was performed for the quantification of IL-6 and TNF-α levels in cell culture supernatant. Results are shown as mean ± S.D. of three independent experiments (#P < 0.05 vs. vehicle-treated control; *P < 0.05 vs. LPS alone).
Effects of BB on COX-2 expression and PGE2 production in LPS- activated RAW264.7 cells
Enhanced COX-2 contributes to the pathophysiological progression of inflammatory disorders (Minghetti, 2004). To investigate the anti-inflammatory effect of BB, Western blotting was performed to evaluate the inhibitory effect of BB on LPS-induced COX-2 expression in RAW 264.7 macrophage. The cells were incubated with different concentration of BB (0.01, 0.5 and 1 ㎎/mL) and treated with LPS for 12 h. As a results, we found that LPS markedly increased the expression levels of COX-2, however, BB attenuated increased COX-2 expression in a concentration-dependent manner (Fig. 2A). PGE2, induced by COX-2, contributes to swelling and pain associated with inflammation. Thus, we assessed whether BB can attenuate the LPS- induced PGE2 production. We observed that PGE2 production were markedly up-regulated in response to LPS, whereas, these increase was significantly alleviated by BB in a concentration-dependent manner (Fig. 2B). The inhibition rates of PGE2 secretion by BB (1 ㎎/mL) was approximately 33.2% (p < 0.05).
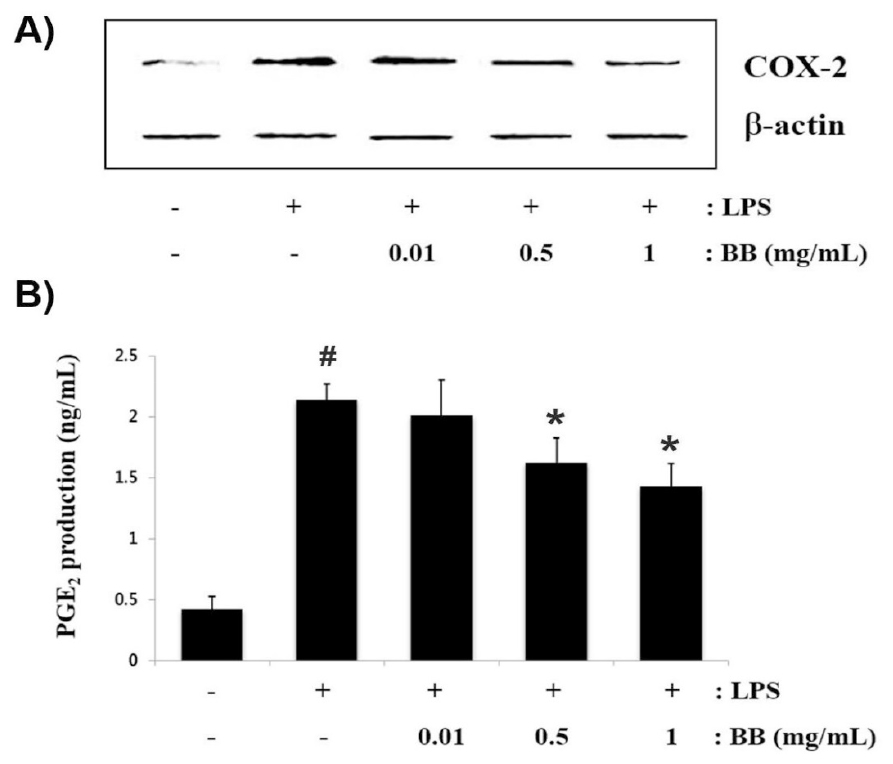
Fig. 2.
Inhibitory effects of BB on LPS-activated COX-2 expression and PGE2 production in RAW264.7 macrophages. (A) Cells (1 × 106 cells/mL) were treated with various dose of BB (0.01 to 1 ㎎/mL) and stimulated with LPS (1 ㎎/mL) for 12 h. The protein extracts were assessed via western blotting to detect of COX-2 levels. (B) The levels of PGE2 secretion in cell culture supernatant was evaluated with colorimetric assay kit, adhering to the manufacturer’s protocol. Results are shown as mean ± S.D. of three independent experiments (#P < 0.05 vs. vehicle-treated control; *P < 0.05 vs. LPS alone).
Effects of BB on IκB-α degradation and NF-κB translocation in LPS- activated RAW264.7 cells
The NF-κB signaling pathway plays an important role in inflammatory process. Especially, phosphorylation and degradation of IκB-α is very important for inducible NF-κB activation and inflammatory regulation (Majdalawieh and Ro, 2010). To investigate the molecular mechanisms by which BB modulate LPS-activated inflammatory responses, we evaluated whether BB modulate NF-κB pathway. Since NF-kB activation requires the IκB-α degradation and translocation of NF-kB into the nucleus, we examined the effects of BB on cytosol IκB-α levels and the nuclear NF-kB levels by western blot analysis. We observed that LPS treatment effectively induce IκB-α degradation in cytosol and the NF-kB translocation into the nuclear, but BB significantly attenuated this enhanced IκB-α degradation and NF-kB translocation in RAW 264.7 cells (Fig. 3A). Relative levels of IκB-α and NF-kB are shown in Fig. 3B.

Fig. 3.
Inhibitory effects of BB on LPS-activated IkB-α degradation and NF-kB activation in RAW264.7 macrophages. (A) Cells (3 × 106 cells/mL) were treated with different dose of BB (0.5, and 1 ㎎/mL) and incubated with LPS (1 ㎎/mL) for 2 h. Cytosol and nuclear extracts were isolated by nuclear extraction kit and western blot analysis was utilized to determine for IκB-α and NF-kB levels. (B) Relative levels of IκB-α in cytosol and NF-kB in nuclear are shown. Results are shown as mean ± S.D. of three independent experiments (#P < 0.05 vs. vehicle-treated control; *P < 0.05 vs. LPS alone).
Effect of BB on caspase-1 activation in LPS- activated RAW264.7 cells
Caspase-1 suppression is a novel therapeutic target for treatment of inflammatory disorders (Guan et al., 2020). Therefore, to further clarify the beneficial mechanism of BB on inflammatory progress, we investigated whether BB suppress the caspase-1 activation in LPS-activated cells. As a results, it was confirmed that LPS enhanced the caspase-1 activity, whereas the increase of caspase-1 activity was down-regulated by BB in a concentration-dependent manner (Fig. 4). The maximal inhibition of caspase-1 activity by BB (1 ㎎/mL) was approximately 34.6% (p < 0.05).

Fig. 4.
Inhibitory effects of BB on LPS-activated caspase-1 activation in RAW264.7 macrophages. (A) Cells (3 × 106 cells/mL) were treated with different dose of BB (0.01, 0.5, and 1 ㎎/mL), and treated with LPS (1 ㎎/mL). Caspase-1 colorimetric assay kit was conducted for quantification of caspase-1 activity, adhering to manufacturer’s protocols. Results are shown as mean ± S.D. of three independent experiments (#P < 0.05 vs. vehicle-treated control; *P < 0.05 vs. LPS alone).
Discussion
Recently, BB is widely consumed worldwide for their richness in highly valuable bioactive compounds particularly phenolic and flavonoids compounds. BB, one of superfoods, has many health-improving activities including anti-oxidant, anti-obesity and anti-cancer property (Kausar et al., 2012; Skrovankova et al., 2015). Although BB has a diverse range of pharmacological actions, its accurate anti-inflammatory mechanism is not well characterized. In this current study, our works demonstrated the anti-inflammatory properties and mechanisms of BB in LPS-stimulated RAW264.7 macrophages.
Inflammation is a complicated process that protect our bodies by neutralizing dangerous germs and preventing infection and wound healing (Christiaens et al., 2008). However, prolonged and excess inflammatory responses can lead to the development of chronic diseases. LPS is one of the most effective macrophage activators and LPS-activated macrophages contribute to inflammatory progress by increasing inflammatory mediators (Liu et al., 2014). In reaction to inflammation, excessive inflammatory cytokines often aggravate and cause tissue damage (Du et al., 2015; Fortunato et al., 2002). Therefore, modulation of macrophage-mediated inflammatory cytokines is a beneficial strategy to prevent further deterioration of tissues. Thus, we explore whether the anti-inflammatory properties of BB were mediated through the suppression of inflammatory cytokines. As a result, the results showed that BB significantly attenuated LPS-induced IL-6 and TNF-α production. The inhibition rates of IL-6 and TNF-α by BB (1 ㎎/mL) were approximately 36.4% and 37.7%, respectively. By virtue of its ability to suppress the increased inflammatory cytokines, BB inhibited the ensuing inflammatory response.
PGs are lipid mediators that regulate physiologic processes in inflammatory conditions (Harris et al., 2002). The overproduction of PGE2, generated by COX-2, is associated to the advancement of inflammatory diseases (Minghetti, 2004). Thus, the modulation of COX-2 expression can be a novel strategy for the management of inflammation-related diseases. In our study, we observed that BB inhibited LPS-enhanced COX-2 expression and PGE2 production. The inhibition rates of PGE2 by BB (1 ㎎/mL) were approximately 33.2%. Based on these finding, we suggested that BB may contribute to alleviating inflammation due to the inhibition of inflammatory mediators.
The NF-κB pathway mediate inflammatory process (Ghosh and Hayden, 2008). Both phosphorylation and degradation of IкB-α play a role in NF-кB activation. During inflammation, IκB-α is phosphorylated and then degraded to induce its dissociation of NF-κB dimers. The free NF-κB migrates to the nucleus, where it controls numerous inflammatory factors (Majdalawieh and Ro, 2010). Attenuation of inflammation is linked to the reduction of NF-кB activation (Celec, 2004). Clinical studies have revealed the overexpression of NF-кB in inflammation tissue from patients. Therefore, modulating NF-κB pathway may be helpful in developing a therapeutic target against inflammatory diseases. Our findings herein discovered that BB significantly attenuated this LPS-enhanced IκB-α degradation in cytosol and NF-kB translocation in nucleus. We predicted that the anti-inflammatory mechanism of BB may be attributed to the blocking of NF-kB pathway.
Accumulating evidence demonstrated that the caspase-1 regulates the inflammatory genes expression (Jeon et al., 2021). Other study have shown that caspase-1 inhibitor attenuated the inflammatory response via down-regulation of NF-κB activation, indicating that caspase-1 is an upstream of NF-κB (Moon and Kim, 2011). For this reason, suppression of NF-kB and caspase-1 activation is an excellent strategy against treating inflammatory-related disorders (Guan et al., 2020). Therefore, to clarify the beneficial mechanism of BB on inflammatory progress, we assessed whether BB regulated the caspase-1 activation in LPS-activated cells. As a results, it was confirmed that BB down-regulated LPS- enhanced the caspase-1 activity in a concentration-dependent manner. The inhibition rates of PGE2 by BB (1 ㎎/mL) were approximately 34.6% (p < 0.05). From this, we hypothesized that anti-inflammatory mechanism of BB is due to the down-regulation of caspase-1 activity in activated macrophages.
In conclusion, this work confirmed that the anti-inflammatory properties of BB may be attributed to the alleviation of the production of IL-6, TNF-a, and PGE2 as well as the expression of COX-2. Additionally, we showed that the anti-inflammatory mechanism of BB is caused by the alleviation of NF-kB pathway and caspase-1 activation in activated macrophages. Collectively, our results conclusively suggested that BB may be a useful candidate for anti-inflammatory therapy.




