Introduction
Materials and Methods
Reagents
Preparation of UP extract
Cell culture
Cell viability test
Cytokine assay
Histamine assay
Western blot analysis
Luciferase reporter gene assay
Caspase-1 activity assay
Statistical analysis
Results
Effect of UP on cell viability in HMC-1 cells
Effect of UP on PMACI-induced inflammatory cytokines levels in HMC-1 cells
Effect of UP on PMACI-induced histamine release in HMC-1 cells
Effect of UP on PMACI-induced NF-κB activation in HMC-1 cells
Effect of UP on PMACI-induced caspase-1 activation in HMC-1 cells
Discussion
Introduction
The inflammatory response involves multiple factors within a complex network. Leukocytes entering the inflammation site are crucial in inflammatory conditions (Hawiger, 2001). In the presence of infection, it products may activate resident lymphocytes and macrophages. Mast activation performs an important role in the inflammatory process (Caughey, 2016; Olivera et al., 2018). In response to various stimuli, mast cell generates a variety of inflammatory mediators, including interleukin (IL)-6, IL-8 and tumor necrosis factor (TNF)-α. The increase of these factors may be critical in inflammatory disorder development (Jennifer, 2018; Yao and Narumiya, 2019). Therefore, inflammatory cytokine suppression may contribute to the development of a useful therapeutic strategy against inflammatory diseases.
Nuclear factor-kB (NF-κB) is a transcription factor that plays a crucial role in regulating the expression of various genes involved in immune and allergic inflammation (Gilmore and Garbati, 2011). Increased NF-κB activity associated with the secretion of high levels of IL-6 and TNF-a is involved in skin inflammation (Gadaleta et al., 2011). Emerging evidence has shown that NF-κB activation and the subsequent increase in inflammatory mediator expression are important in inflammatory diseases (Birrell et al., 2005). Caspase-1 is a member of the caspases family and is associated with inflammatory reaction. It is well established that caspase-1 activation induces an inflammatory response by increasing inflammatory mediators and inflammatory cells recruitment (Kim et al., 2016). These results demonstrate that caspase-1 as an attractive target for inflammatory diseases treatment.
Recently, an increasing number of studies have shown that marine source have various pharmacological applications. Therefore, they are widely used as healthcare products worldwide. Undaria pinnatifida (UP), edible brown algae, reportedly has several biological activities, including anti-tumor, anti-oxidative, anti-viral, and anti-angiogenic properties (Etman et al., 2021; Lee et al., 2020; Yoshinaga and Mitamura, 2019). However, information on the precise mechanism of UP on mast cell-mediated inflammatory response remains limited. We investigated the effect of UP on the inflammatory cytokines production and NF-κB/caspase-1 activation in stimulated human mast cells (HMC-1) to elucidate a possible explanation for anti-inflammatory mechanisms of UP.
Materials and Methods
Reagents
The following chemicals were procured from Sigma (St. Louis, MO, USA): bicinchoninic acid (BCA), dimethyl sulfoxide (DMSO), calcium Ionophore A23187, avidin peroxidase (AP), phorbol 12-myristate 13-acetate (PMA) and 3-(4,5- dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) from the Sigma Chemical Co. (St. Louis, MO, USA). Iscove's Modified Dulbecco's Medium (IMDM) was obtained from Gibco BRL (Grand Island, NY, USA). The ELISA assay kits for IL-6/ IL-8/TNF-α were purchased from BD Biosciences (San Diego, CA, USA). Specific antibodies against NF-κB and histone Abs were procured from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Preparation of UP extract
Dried UP was purchased from a local market (Wando, Korea) and authenticated by Dr. En-Mi Ahn (Daegu Haany University). The dried UP (100 g) was pulverized into a fine powder and extracted in 1 L of a 70% aqueous ethanol solution at room temperature for 24 h, and then concentrated under vacuum. The extract was filtered, freeze drying (Ilshinbiobase, ILSHINBIOBASE-FD, Korea) and kept at 4℃(yield, 11.8%). The sample was dissolved in PBS and filtered through a 0.22 ㎛ syringe filter (GVS ABLUO, Fisher scientific, USA).
Cell culture
Cells were maintained in IMDM containing with penicillin (100 IU/ml), streptomycin (100 mg/ml), and 10% FBS at 37℃in 5% CO2 atmosphere. HMC-1 was stimulated with of PMA (50 nM) plus A23187 (1 ㎎/mL).
Cell viability test
To investigate the cell viability at various concentrations of UP, the MTT colorimetric assay was conducted (Jung et al., 2014; Kim et al., 2021). Briefly, Cells were treated with UP (0.1, 0.5 and 1 ㎎/mL) for 24 h and MTT (50 μL) was subsequently added. After incubation for 4 h, the crystallized formazan was dissolved in DMSO and the absorbance was measured at 540 ㎚ by a microplate reader (Molecular Devices, CA, USA).
Cytokine assay
The levels of IL-6, IL-8 and TNF-α was evaluated by modified enzyme-linked immunosorbent assays (ELISA) as previously described (Kim et al., 2010). Briefly, micro plates (96-well) were coated with anti-human IL-6, IL-1β, IL-8 and TNF-α monoclonal Abs and incubated overnight at 4℃. After additional washes, the samples or IL-6, IL-1β, IL-8 and TNF-α standard solution was added and incubated. After additional washing, the plates were incubated to biotinylated Abs and incubated for 2 h. After washing the plates, AP and ABTS substrates were sequentially added, and then color development was read at 405 ㎚.
Histamine assay
Histamine concentration derived from mast cell was determined with a histamine assay kit according to the manufacturer’s protocol (Neogen, Lexington, USA).
Western blot analysis
Nuclear lysates were prepared by nuclear extraction reagent kit (Pierce Thermo Scientific, Rockford, IL, USA) as previously described (Nam et al., 2017; Yu et al., 2021). After protein quantification, the sample was mixed with 2x sample buffer, separated by gel electrophoresis and transferred to membranes. Membrane was blocked with skimmed milk (5%) and subsequently reacted with NF-κB p65 primary Abs. After washing, the membrane was incubated with secondary Abs for 1 h. After washing, protein bands were visualized using an enhanced chemiluminescence detection system (Thermo Fisher Scientific Inc. NJ, USA).
Luciferase reporter gene assay
NF-κB promoter activity was determined using luciferase assay as previously described (Sakamoto et al., 2018). Briefly, cells were transfected by NF-κB-luc DNA and changed with media. The transfected cells were seeded in plates and treated with UP before stimulation for another 2 h. The activity of luciferase was determined using the Dual-Glo Luciferase Assay System according to manufacturer’s protocols (Promega, WI, USA).
Caspase-1 activity assay
Caspase-1 activity was measured by a caspase-1 colorimetric assay kit (R&D System Inc., Minneapolis, MN, USA) following the manufacturer's protocols. After protein quantification, the sample was reacted with reaction buffer (50 μL) and caspase-1 substrate (5 μL) at 37℃ for 2 h. The absorbance was assessed at 405 ㎚ by a microplate reader.
Statistical analysis
The results are presented as the mean ± S.D. The statistical analyses were performed using an independent t- test and ANOVA with a Tukey post hoc test. P < 0.05 was considered significant.
Results
Effect of UP on cell viability in HMC-1 cells
First, we elucidated the cytotoxic effect of UP to elucidate its anti-inflammatory effect and mechanism. The cells were pretreated with or without UP (0.1, 0.5 and 1 ㎎/ mL) for 1 h before stimulation with PMACI for 24 h, and the cytotoxic effects of UP was evaluated using an MTT assay. The result showed that there was no cell cytotoxicity due to UP (Fig. 1).
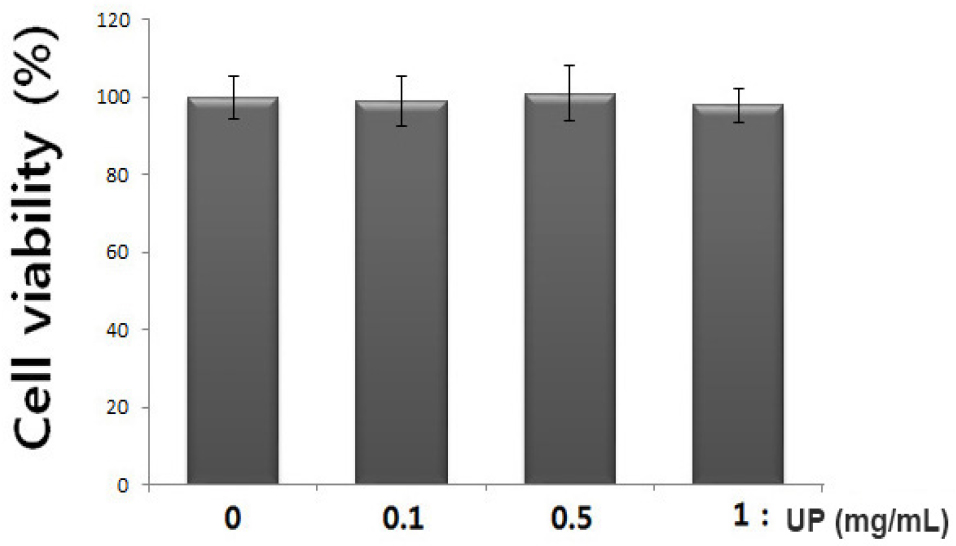
Fig. 1.
Effect of UP on cell viability in stimulated- HMC-1 cells. (A) Cells (3 × 105cells/well) were treated with various concentration of UP (0.1, 0.5 and 1 ㎎/ mL) for 24 h and cell viability was measured using the MTT assay. The values are represented as the mean ± S.D. of independent experiments (#P < 0.05 vs. control, *P < 0.05 vs. PMACI alone treatment).
Effect of UP on PMACI-induced inflammatory cytokines levels in HMC-1 cells
Inflammatory cytokine level attenuation is a therapeutic strategy for inflammatory disease (Ogata and Hibi, 2003). Therefore, we evaluated the inhibitory effect of UP on IL-6, IL-8 and TNF-α secretion in PMACI-stimulated HMC-1 cells. The cells were treated with or without UP (0.1, 0.5 and 1 ㎎/ mL) for 1 h before stimulation with PMACI for 8 h. The results showed that PMACI alone induced IL-6, IL-8 and TNF-α production in PMACI-stimulated HMC-1cells. However, UP attenuated the PMACI-induced increase IL-6, IL-8 and TNF-α secretion in a dose-dependent manner (Fig. 2). Inhibitory rate of UP on inflammatory cytokine production has been presented in Table 1. The maximal inhibition of IL-6, IL-8 and TNF-α secretion by UP (1 ㎎/mL) was approximately 44.05% (p < 0.05), 40.83% (p < 0.05), and 46.07% (p < 0.05), respectively.
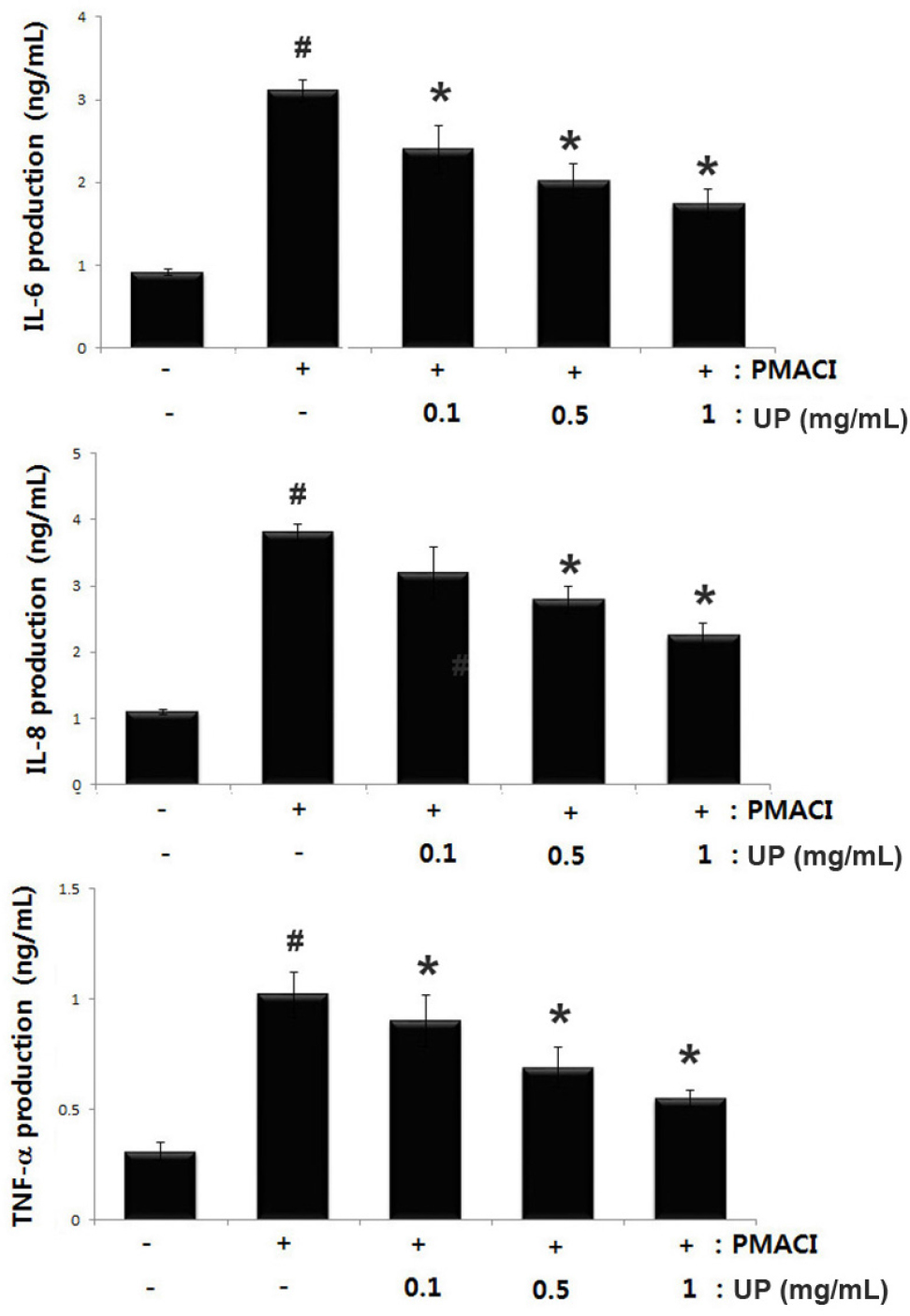
Fig. 2.
Effects of UP on PMACI-induced IL-6, IL-8 and TNF-α production in stimulated- HMC-1 cells. Cells (3 × 105cells/ well) were treated with various concentration of UP (0.1, 0.5 and 1 ㎎/mL) for 1 h before stimulation with PMACI for 8 h. The IL-6, IL-8 and TNF-α concentration were determined by ELISA kits. The values are represented as the mean ± S.D. of independent experiments (#P < 0.05 vs. control, *P < 0.05 vs. PMACI alone treatment).
Table 1.
Inhibitory rate of UP on inflammatory cytokine in stimulated HMC-1 cells
| UP (㎎/mL) | IL-6 (%) | IL-8 (%) | TNF-a (%) |
| 0.1 | 22.82±2.01* | 16.01±7.51 | 11.78±3.32* |
| 0.5 | 35.05±3.28* | 26.77±4.02* | 32.35±4.05* |
| 1 | 44.05±2.91* | 40.83±2.14* | 46.07±2.12* |
Effect of UP on PMACI-induced histamine release in HMC-1 cells
Mast cell-derived histamine initiates allergic inflammation and their appropriate regulation could help treat allergic inflammatory disease (Caughey, 2016). Therefore, we measured the regulatory effect of UP on PMACI-induced histamine release using histamine assay kit. UP decreased PMACI- induced histamine levels in a dose-dependent manner (Fig. 3), suggesting that UP exerts an anti-allergic inflammatory effect through inhibition of histamine release. Inhibitory rate of UP on histamine release has been shown in Table 2.
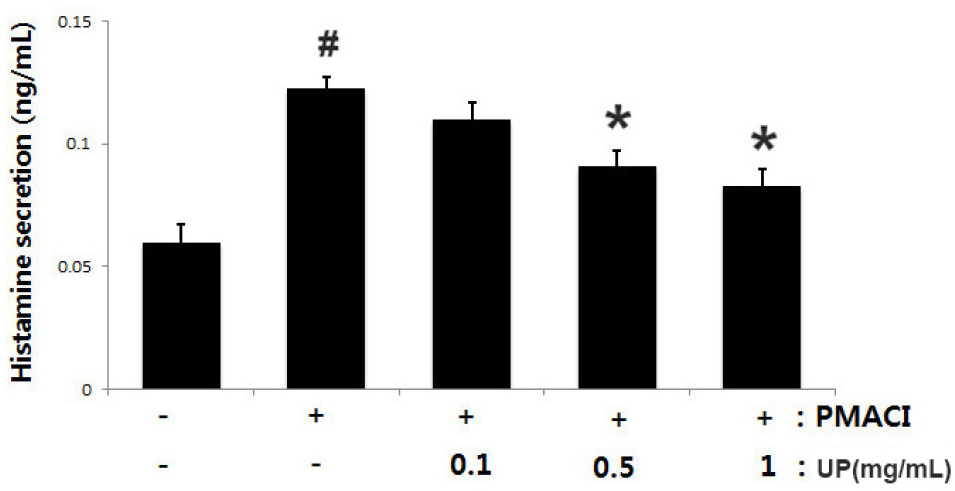
Fig. 3.
Effects of UP on PMACI-induced histamine levels in stimulated- HMC-1 cells. Cells (1 × 106cells/well) were treated with various concentration of UP (0.1, 0.5 and 1 ㎎/mL) for 1 h before stimulation with PMACI for 1 h. The histamine concentration was measured by histamine assay kit. The values are shown as the mean ± S.D. of independent experiments (#P < 0.05 vs. control, *P< 0.05 vs. PMACI alone treatment).
Table 2.
Inhibitory rate of UP on histamine release in stimulated HMC-1 cells
| UP (㎎/mL) | Inhibitory rate of Histamine (%) |
| 0.1 | 10.50±5.87 |
| 0.5 | 26.10±2.26* |
| 1 | 32.61±2.07* |
Effect of UP on PMACI-induced NF-κB activation in HMC-1 cells
As NF-κB suppression has been linked to anti-inflammatory response, we predicted that NF-κB activation attenuation might be the molecular mechanism of UP. Since NF-κB activation requires nuclear NF-κB translocation, we investigated the effects of UP on these processes using Western blot analysis. Fig. 4A shows PMACI- induced NF-κB translocation in the nucleus compared to the control. UP attenuated enhanced NF-κB translocation. The NF-κB relative level is represented (Fig. 4B). Additionally, we evaluated the effect of UP on NF-κB the promoter activity using a luciferase reporter assay. As Fig. 4C illustrates, UP inhibited PMACI-induced NF-κB- driven luciferase activity.
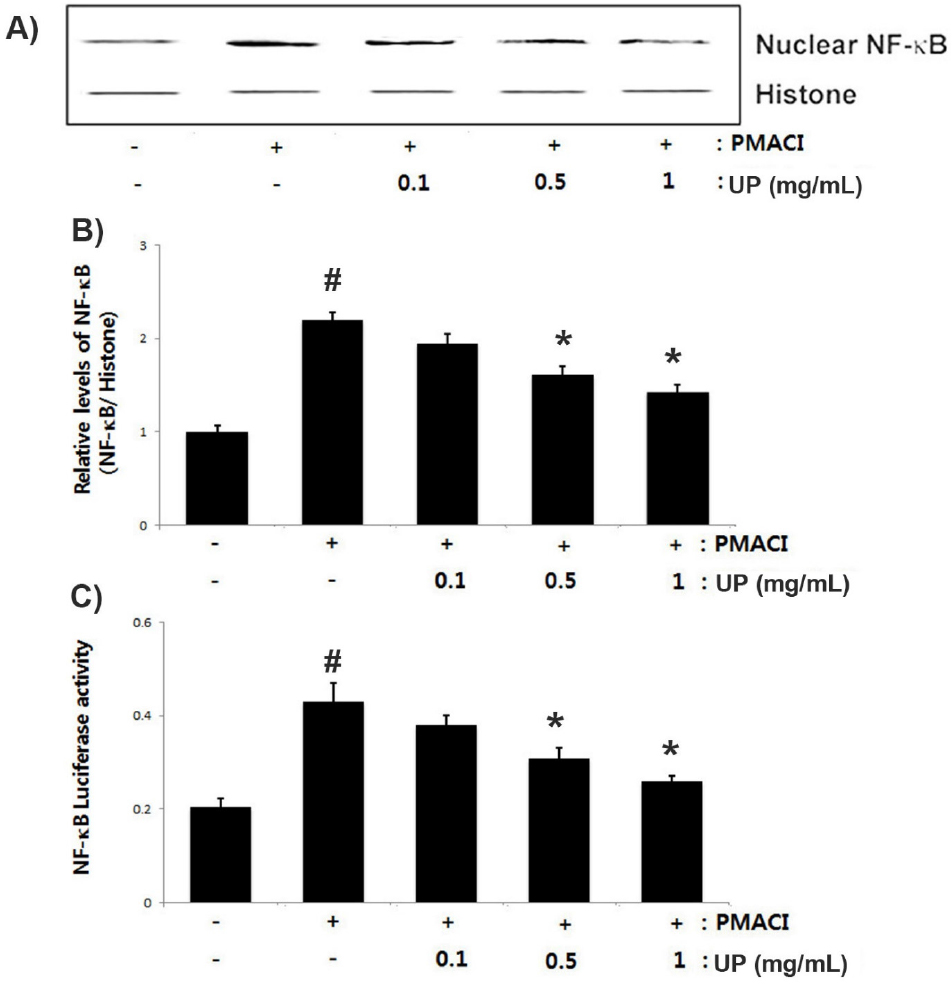
Fig. 4.
Effect of UP on PMACI-induced NF-κB activation in stimulated- HMC-1 cells. Cells (5 × 106 cells/well) were treated with various concentration of UP (0.1, 0.5 and 1 ㎎/mL) for 1 h and then stimulated with PMACI for 2 h. (A) Nuclear extracts were prepared and evaluated for NF-κB (RelA/p65) expression by Western blot analysis. (B) NF-κB relative level is presented. (C) NF-κB the promoter activity was determined using a luciferase assay kit following the manufacturer’s protocols. The values are represented as the mean ± S.D. of independent experiments (#P < 0.05 vs. control, *P < 0.05 vs. PMACI alone treatment).
Effect of UP on PMACI-induced caspase-1 activation in HMC-1 cells
Han et al. (2017) reported that the caspase-1 activation is related to an inflammatory response via an increase in inflammatory cytokines in mast cell. We investigated the effects of UP on caspase-1 activity using a caspase-1 assay kit to determine the molecular mechanism of UP involvement in the inflammatory response. The results showed that the PMACI- induced caspase-1 activity was significantly inhibited by UP in a dose-dependent manner (Fig. 5). Inhibitory rate of UP on caspase-1 activity has been represented in Table 3. The maximal inhibition of caspase-1 activity by UP (1 ㎎/mL) was approximately 35.45% (p < 0.05).
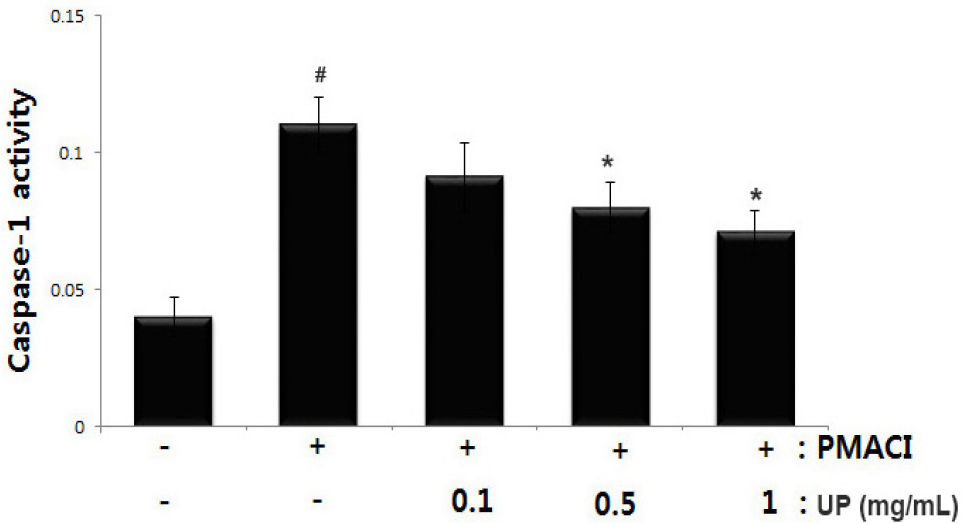
Fig. 5.
Effect of UP on PMACI-induced caspase-1 activation in HMC-1. Cells (5 × 106 cells/well) were pretreated with UP (0.1, 0.5 and 1 ㎎/mL) for 1 h before stimulation with PMACI for 4 h. The caspase-1 enzymatic activity was measured using a colorimetric assay kit. The values are represented as the mean ± S.D. of independent experiments (#P < 0.05 vs. control, *P < 0.05 vs. PMACI alone treatment).
Discussion
Marine sources as potential treatment options for various diseases have been a subject of growing interest (Kimur and Maki, 2002; Lee et al., 2020). In this study, we demonstrated the anti-inflammatory effect and molecular mechanisms of UP in an in vitro model of mast cell-mediated allergic inflammation. The findings of this study showed that UP effectively attenuated the PMACI-induced IL-6, IL-8, TNF-α and histamine secretion in HMC-1cells. Additionally, we showed that the inhibitory mechanisms of UP on allergic inflammation arose from NF-κB/caspase-1 suppression in PMACI-stimulated HMC-1cells.
Mast cells play differential roles in inflammation, initiating and regulating immune responses by releasing various cytokines and chemokines via several intracellular signal transduction pathways (Harvima and Nilsson, 2011). In response to different stimuli, mast cells release an array of cytokines with the potential to cause inflammation (Galli et al., 2005). It was been reported that IL-6 causes inflammatory response, activates T-lymphocyte, and produces IgE (Trefzer et al., 2003). Mast cell-derived IL-8 acts as a chemotactic factor for eosinophil and neutrophil, activating inflammatory response. TNF-α secreted from mast cells, accumulates white blood cells, resulting in inflammation response (Fedenko et al., 2011). In this study, we demonstrated that UP attenuated PMACI-induced the production of IL-6, IL-8 and TNF-α in HMC-1 cells. The inhibition rates of IL-6, IL-8 and TNF-α by UP (1 ㎎/mL) were approximately 44.05 %, 40.83 %, and 46.07%, respectively. These results suggest that UP exerts an anti-inflammatory effect by suppressing of inflammatory cytokine production. Mast cell-derived histamine is associated with allergic inflammatory response (Modena et al., 2016). Histamine plays a central role in the pathogenesis of several allergic diseases, such as atopic dermatitis, allergic rhinitis, and allergic asthma through release of leukotrienes, cytokines, and chemokines (Jemima et al., 2014). Therefore, histamine release regulation could help treat allergic inflammatory disease. The results showed that UP decreased PMACI- induced secretion of histamine in a dose-dependent manner (Fig. 3). The maximal inhibition of histamine secretion by UP (1 ㎎/mL) was approximately 32.60% (p < 0.05). This suggests that UP displays anti-allergic inflammatory activity, inhibiting histamine release.
An increase in inflammatory mediators is associated with NF-κB activation (Tak and Firestein, 2001). In inflammatory process, the IκB kinase complex phosphorylates and degrades the IκB proteins and NF-κB is translocate into the nucleus, where it combines to the promoter and activate target gene expression (Birrell et al., 2005). Recent progress has suggested that caspase-1 activation plays a critical role in regulating the innate immune system (Martin et al., 2013). Emerging evidence has shown that caspase-1 activation exerts its biological activities by inducing various inflammatory pathways (Han et al., 2017). Caspase-1 deficiency has been reported to inhibit the inflammatory reaction in an allergic rhinitis mice model (Nam et al., 2014). These studies demonstrated NF-κB/caspase-1 inhibition as an anti-inflammatory strategy. Therefore, to identify the anti-inflammatory mechanism of UP, we investigated whether UP could attenuated the PMACI induced the activation of NF-κB/caspase-1 in HMC-1 cells. Our results showed that UP inhibited PMACI- induced the NF-κB translocation into the nucleus and NF-κB the promoter activity. Additionally, UP attenuated the PMACI- induced caspase-1 activation in a dose-dependent manner. Taken together, our results indicated that the regulatory effect of UP on mast cell-mediated inflammation might be resulting from the blockage of NF-κB/caspase-1 activation in stimulated- HMC-1 cells.
In conclusion, the anti-inflammatory effect of UP can attributed to inflammatory cytokine regulation in PMACI- stimulated HMC-1 cells. Additionally, we demonstrated that the mast cell-mediated anti-inflammatory effect of UP results from NF-κB/caspase-1 activation attenuation. These results provide experimental evidence that UP might be potential candidate for inflammation- related diseases treatment.




